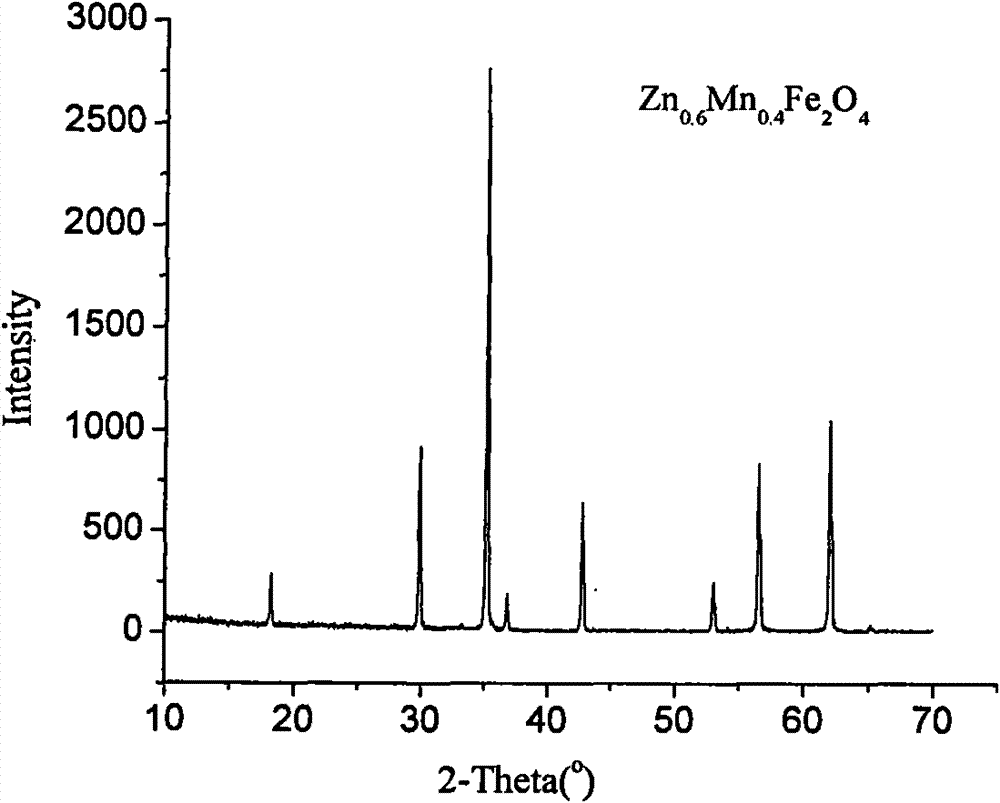
Synthesis method of Mn-Zn ferrite crystal
A manganese-zinc ferrite and synthesis method technology, applied in the field of synthesis of high-performance manganese-zinc ferrite crystals, can solve problems such as difficult large-scale production, harsh synthesis conditions, uneven mixing, etc., and overcome step-by-step precipitation Effects of problems, ease of control, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Take zinc sulfate (ZnSO 4 ·7H 2 O) powder 2.5mol (718.9g), manganese acetate [Mn(CH 3 COO) 2 4H 2 O] powder 7.5mol (1837.8g), ferrous nitrate [Fe(NO 3 ) 2 ·6H 2 O] powder 20mol (5759.0g), sodium carbonate (Na 2 CO 3 10H 2 O) Powder 34.5 mol (9871.8 g), polyethylene glycol-400 (363.8 g). Add sodium carbonate powder into an enamel container, then add polyethylene glycol-400 and mix evenly for later use, then add to the sodium carbonate powder mixed with polyethylene glycol-400 under stirring at room temperature The above-mentioned zinc salt powder, manganese salt powder, and iron salt powder are added and mixed evenly, then moved into the grinding equipment for full grinding for 40 minutes, left at room temperature for 1.5 hours, washed the reaction mixture with water to the filtrate with 0.5mol L -1 BaCl 2 The solution does not detect SO 4 2- filter under reduced pressure. Dry the filter cake at 120°C for 3-4 hours, grind the dried filter cake into powder, ...
Embodiment 2
[0047] Take zinc chloride (ZnCl 2 ) powder 2.5mol (340.73g), manganese sulfate (MnSO 4 4H 2 O) powder 7.5mol (1673.0g), ferrous chloride (FeCl 2 4H 2 O) powder 20mol (3976.2g), sodium carbonate (Na 2 CO 3 10H 2 O) Powder 34.5 mol (9871.8 g), OP-10 (317.2 g). Add sodium carbonate powder into an enamel container, then add OP-10 and grind and mix evenly for later use, then add the above-mentioned zinc salt to the sodium carbonate powder ground and mixed with OP-10 under stirring at room temperature and normal pressure Powder, manganese salt powder, iron salt powder, after adding and mixing evenly, move it into the grinding equipment and grind it fully for 50min, let it stand at room temperature for 1.5h, wash the reaction mixture with water to the filtrate with 0.5mol L -1 BaCl 2 The solution does not detect SO 4 2-filter under reduced pressure. Dry the filter cake at 120°C for 3-4 hours, grind the dried filter cake into powder, and then place it in a high-temperature ...
Embodiment 3
[0049] Take zinc nitrate [Zn(NO 3 ) 2 ·6H 2 O] powder 2.5mol (743.7g), manganese nitrate [Mn(NO 3 ) 2 ·6H 2 O] powder 7.5mol (2152.8g), ferrous sulfate (FeSO 4 ·7H 2 O) powder 20mol (5560.2g), ammonium carbonate [(NH 4 ) 2 CO 3 ] Powder 34.5mol (3315.1g), Tween-80 (118g), triethanolamine hydrochloride (118g). Add ammonium carbonate powder into an enamel container, then add half of Tween-80 and half of triethanolamine hydrochloride and mix well, set aside. In another enamel container, fully mix zinc nitrate powder, manganese nitrate powder, and ferrous sulfate powder evenly, then add the other half of Tween-80 and the other half of triethanolamine hydrochloride, mix evenly, and set aside. Then at room temperature and normal pressure, add the above-mentioned mixture of zinc salt powder, manganese salt powder, and iron salt powder to the ammonium carbonate powder under stirring. Set aside for 2h, wash the reaction mixture with water to the filtrate with 0.5mol L -1 Ba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com