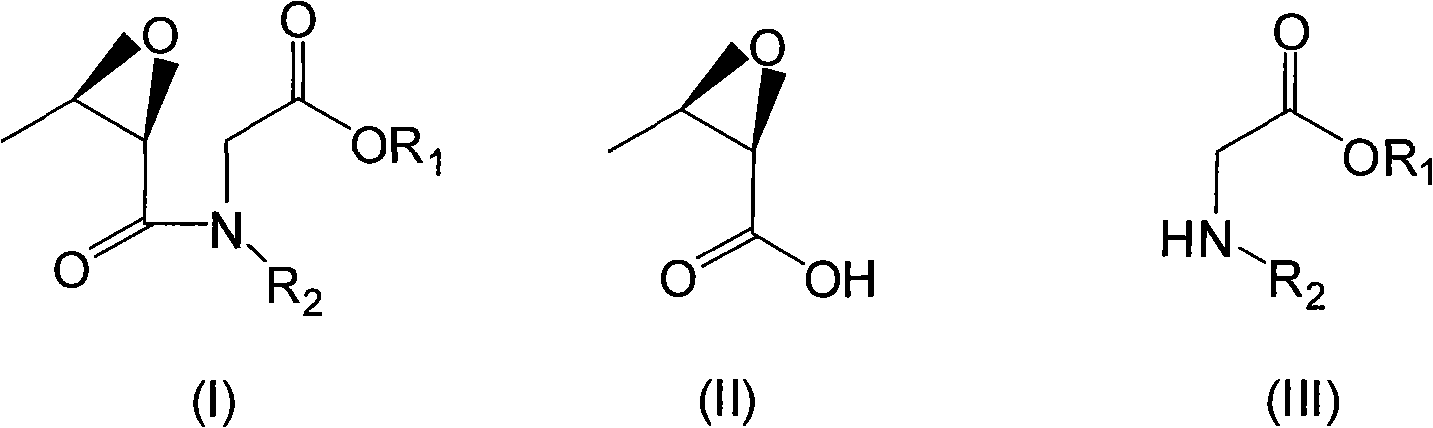
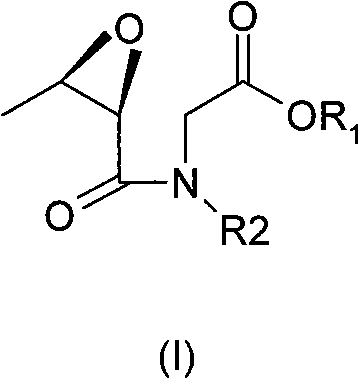
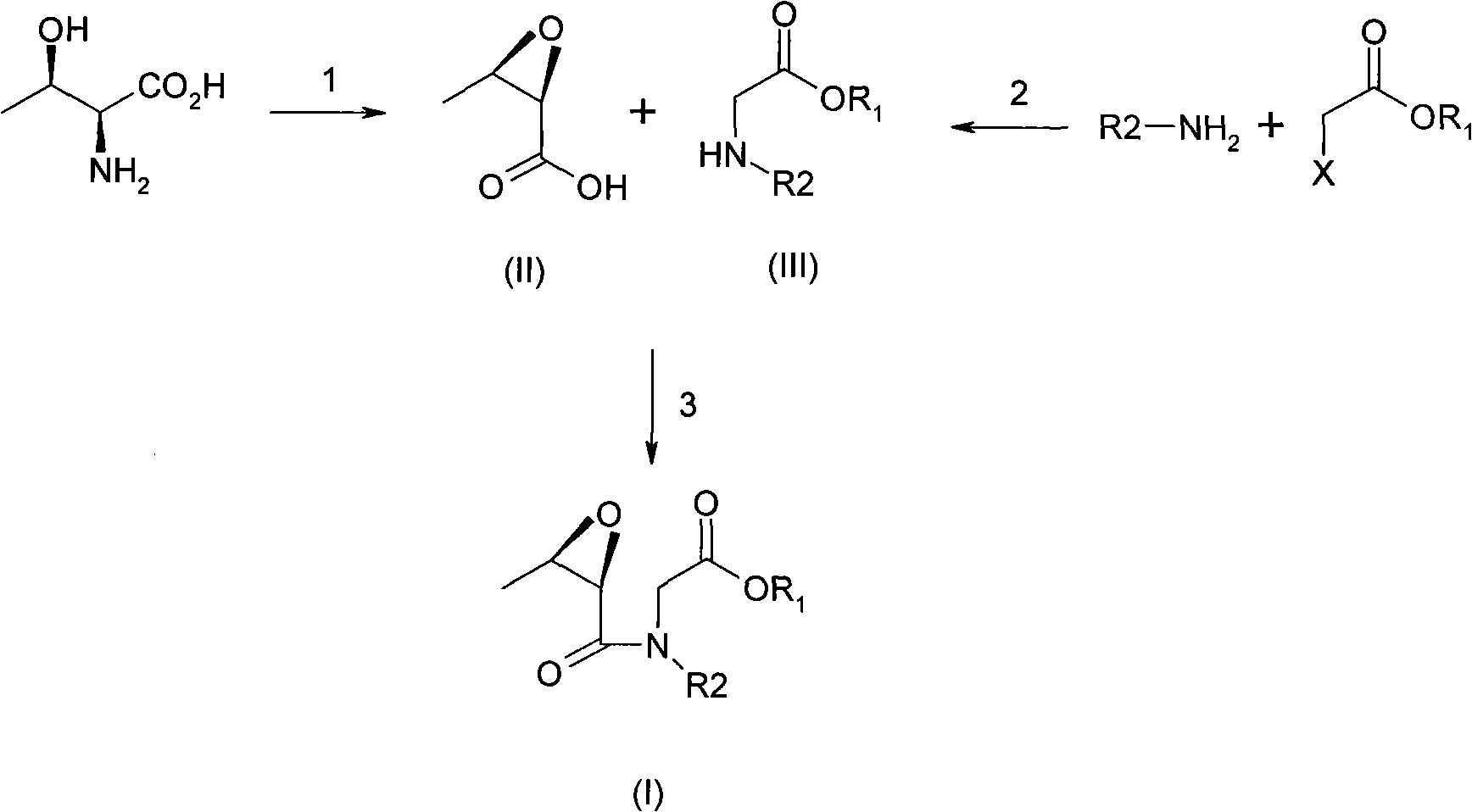
Method for preparing azetidinone derivatives
A technology of epoxy butyric acid and epoxy butyramide, applied in the direction of organic chemistry and the like, can solve problems such as unfavorable industrial production, high cost of acid binding agent, low reaction temperature, etc., and achieves simplified operation, reduced cost, and increased reaction temperature. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1. (2R, 3S)-N-(ethoxycarbonyl)methyl-N-p-methoxyphenyl-2,3-epoxybutanamide
[0020] In a 500 ml four-necked flask, 10.2 g (0.1 mol) of (2R, 3S)-epoxybutyric acid was added and dissolved in 200 ml of ethyl acetate. Cool to 0°C-5°C, add 12.5g (0.134mol) of 4-methylpyridine dropwise at this temperature, then add 12.5g (0.104mol) of pivaloyl chloride dropwise, and keep warm for 2.5hr after dropping. Then, 18.5 g (0.089 mol) of ethyl N-(4-methoxyphenyl) acetate was charged and reacted at this temperature for 1 hr. Then the temperature was raised to 20° C. to 25° C., and the reaction was carried out for 16 hours, and the reaction progress was detected by HPLC. After the reaction, the mixture was washed with dilute hydrochloric acid, saturated sodium bicarbonate, and saturated brine, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain an oil. Then recrystallized from diethyl ether to obtain 23.5 g of the title compo...
Embodiment 2
[0021] Example 2. (2R, 3S)-N-(ethoxycarbonyl)methyl-N-p-methoxyphenyl-2,3-epoxybutanamide
[0022] In a 500 ml four-necked flask, 10.2 g (0.1 mol) of (2R, 3S)-epoxybutyric acid was added and dissolved in 200 ml of ethyl acetate. Cool to 0°C-5°C, add 15g (0.148mol) triethylamine dropwise at this temperature, and then add 12.5g (0.104mol) pivaloyl chloride dropwise, and keep warm for 1hr after dropping. Then, 18.5 g (0.089 mol) of ethyl N-(4-methoxyphenyl) acetate was charged and reacted at this temperature for 1 hr. Then the temperature was raised to 20° C. to 25° C., and the reaction was carried out for 14 hours, and the reaction progress was detected by HPLC. After the reaction, the mixture was washed with dilute hydrochloric acid, saturated sodium bicarbonate, and saturated brine, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain an oil. Then recrystallized from diethyl ether to obtain 23 g of the title compound. (Yiel...
Embodiment 3
[0023] Example 3. (2R, 3S)-N-(ethoxycarbonyl)methyl-N-p-methoxyphenyl-2,3-epoxybutanamide
[0024] In a 500 ml four-neck flask, 10.2 g (0.1 mol) of (2R,3S)-epoxybutyric acid was added and dissolved in 200 ml of chloroform. Add 10.6 g (0.134 mol) of pyridine dropwise at a temperature of 20° C. to 25° C., and then add 12.5 g (0.104 mol) of pivaloyl chloride dropwise, and keep warm for 0.5 hr after dropping. Then, 18.5 g (0.089 mol) of ethyl N-(4-methoxyphenyl) acetate was added and reacted at this temperature for 1.5 hr. Then the temperature was raised to 30° C. to 35° C., and the reaction was carried out for 16 hours, and the reaction progress was detected by HPLC. After the reaction was completed, the mixture was washed with dilute hydrochloric acid, saturated sodium bicarbonate, and saturated brine, dried over anhydrous sodium sulfate, and evaporated to remove the solvent under reduced pressure to obtain an oil. Then, it was recrystallized from hexane to obtain 23.3 g of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com