Treatments and diagnostics for cancer, inflammatory disorders and autoimmune disorders
An active, selected technology, applied in the field of tumor growth, which can solve the problems of impaired antigen presentation and incomplete maturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] Protein variants are prepared optionally by site-directed mutagenesis of nucleotides in the DNA encoding the native protein or by phage display techniques, whereby DNA encoding the variant is generated, which is then expressed in recombinant cell culture.
[0072] Although the sites for introducing amino acid sequence variations are predetermined, the mutations themselves need not be predetermined. For example, to optimize the consequences of a mutation at a given site, random mutagenesis can be performed at a codon or region of interest and the expressed protein variants screened for the best combination of desired activities. Techniques for substitutional mutagenesis at predetermined sites in DNA of known sequence are well known, such as, for example, site-specific mutagenesis. Production of protein variants described herein can be accomplished by phage display techniques, such as those described in PCT publication WO 00 / 63380.
[0073] After such clones are selected...
Embodiment 1
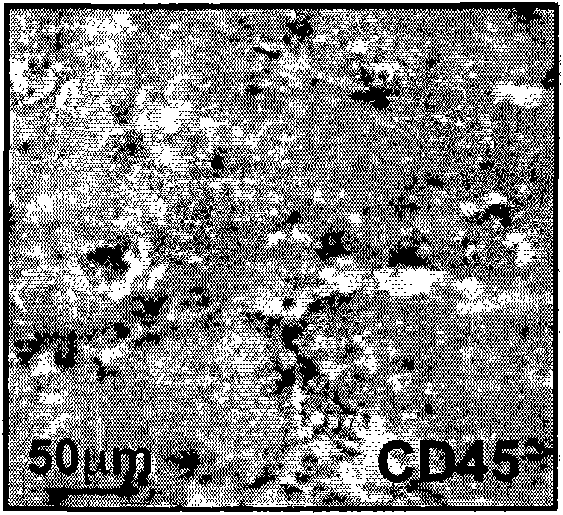
[0334] Example 1: Composition and Localization of Myeloid Infiltrates
[0335] The composition and localization of immune infiltrates in MMTV-PyMT-induced breast tumors were assessed by immunohistochemistry. Wild-type mice (Charles River) susceptible to Friend leukemia virus strain B ("FVB") were purchased and raised in a pathogen-free facility containing MMTV.PyMT in a FVB background tg or MMTV.Her2 tg Tumor mice. will come from MMTV.PyMT tg Tumors from mice were embedded and frozen in OCT solution. Using standard protocols, cryosections were cut into 5 micron thin sections, dried at room temperature, and fixed with ice-cold acetone. Endogenous peroxidase was quenched with glucose oxidase for 60 minutes at 37°C. Sections were rinsed with PBS and endogenous avidin and biotin blocked with the avidin biotin blocking kit (Vector) according to the manufacturer's instructions. Sections were blocked with 10% rabbit serum in 3% BSA / PBS for 30 minutes at room temperature, then i...
Embodiment 2
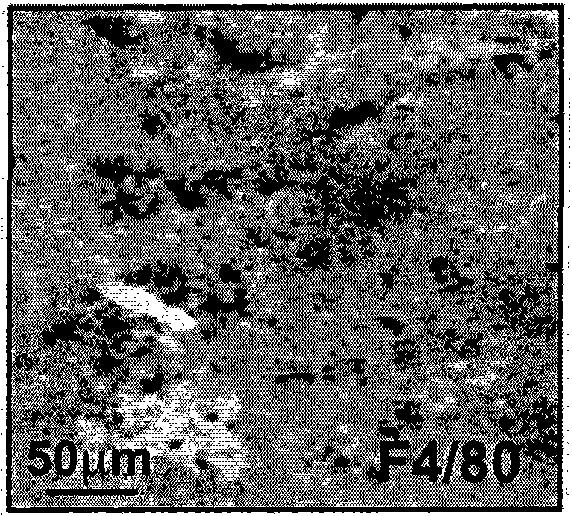
[0342] Example 2: Characterization of TAMs
[0343] A. TAMs express CD11c and Langerin and exhibit characteristics of professional antigen-presenting cells
[0344] Both macrophages and dendritic cells ("DC") have the ability to capture antigens and present them to T cells. To better understand the role of TAMs in the regulation of T cell responses, the expression of genes often associated with antigen presentation within tumors was assessed. Immunohistochemical analysis of TAMs for markers normally expressed on myeloid or DC cells was performed following the method described in Example 1. Rat anti-F4 / 80 antibody was obtained from Serotec, rat anti-CD11b antibody from eBioscience and rat anti-CD11c antibody from Pharmingen. Immunohistochemistry against anti-human langerin (CD207) was generally performed as described in Example 1, but the tissue sections were deparaffinized and used in target recovery buffer (pH 6.0, Dako Cytomation) at 99 Antigen retrieval was carried out a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com