Amino-terminated polypropylene and preparation method thereof
A technology of amino-terminated polypropylene and hydroxyl-terminated polypropylene, which is applied in the field of amino-terminated polypropylene and its preparation, and can solve problems such as reduced catalytic polymerization efficiency
Active Publication Date: 2010-08-25
PETROCHINA CO LTD
View PDF1 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the chain transfer agent used in this method usually needs to be specially synthesized, and the addition of the chain transfer agent also reduces the catalytic polymerization efficiency.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2-22
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
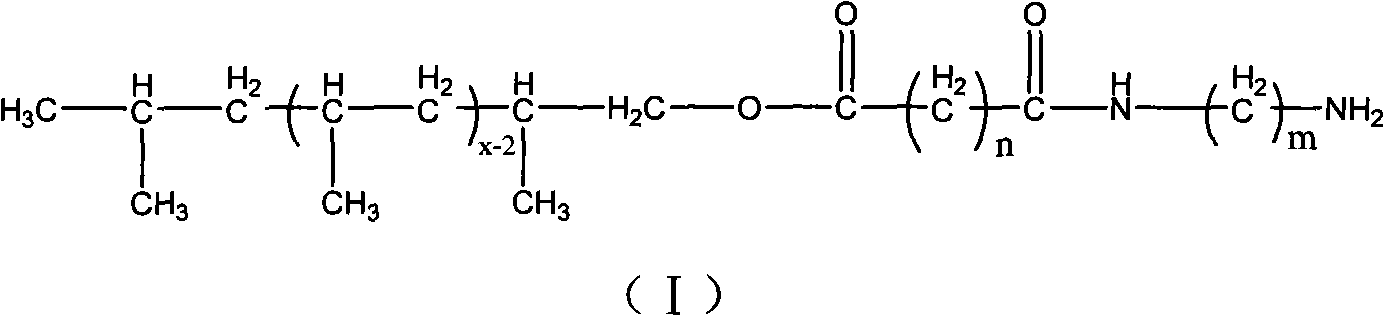
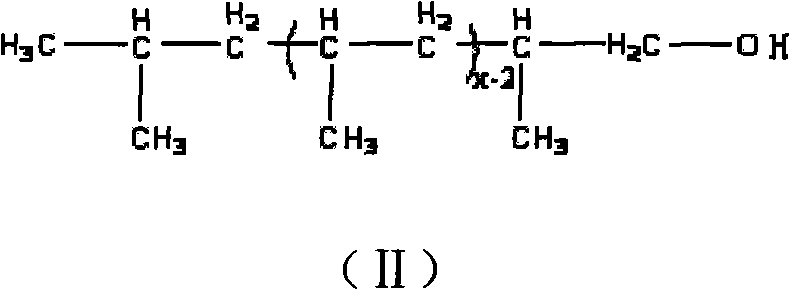
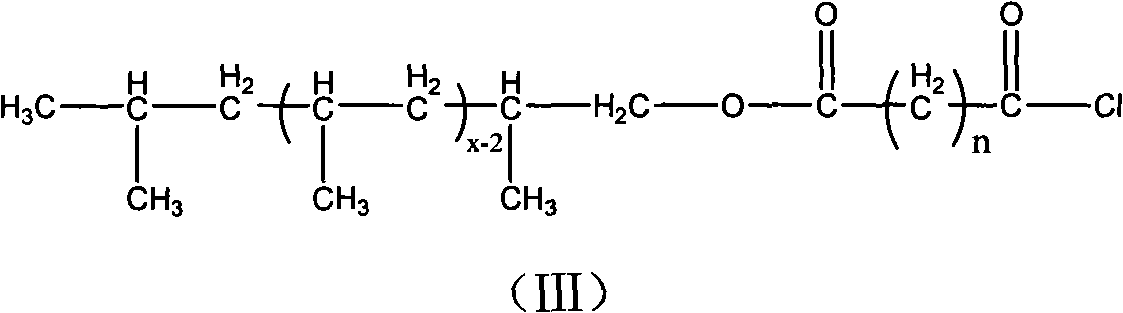
The invention relates to amino-terminated polypropylene, wherein one end of a macromolecular chain carries with an amino group, the structure of the amino-terminated polypropylene accords with to general formula (I). The amino-terminated polypropylene is obtained by reacting a hydroxyl group of hydroxyl-terminated polypropylene with acyl-chlorinated diacid and further reacting the obtained polypropylene which carries an acid acyl chloride group at the terminal with diamine. The amino-terminated polypropylene has strong polarity and has wide application in the fields of polymer blend, composite materials and the like.
Description
Amino-terminated polypropylene and its preparation method technical field The invention relates to an amino-terminated polypropylene and a preparation method thereof. technical background Polyolefin is a material with a wide range of applications, but the molecular chain of polyolefin is completely composed of saturated hydrocarbons and lacks polar functional groups. The adhesion, printing and dyeing properties of polyolefin and the compatibility with other polymers or fillers are poor, which limits the application of polyolefin in many aspects. Therefore, it is of great practical significance to introduce polar functional groups into polyolefins to realize the functionalization of polyolefins. However, the chemical stability of polyolefin chains makes the functionalization of polyolefins more difficult, and the functionalization of polyolefin end groups is more difficult than the functionalization of polyolefin side chains. However, since the terminal functional group o...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C08F110/06C08F8/32
Inventor 贾军纪董金勇朱博超牛慧徐人威王霞黄安平许云波王丹丹任峰孙卫国
Owner PETROCHINA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com