Antibodies binding to an intracellular prl-1 or prl-3 polypeptide
A PRL-1 and antibody technology, applied in the medical field, can solve the problems that antibodies cannot pass through the cell membrane and cannot access antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 27
[0078] Example 27 shows that anti-PRL1 antibody 269 can bind to epitopes TYKNMR and TLNKFI. Therefore, the present inventors provide an anti-PRL antibody such as an anti-PRL1 antibody capable of binding to the sequence TYKNMR. The present inventors also provide anti-PRL antibodies such as anti-PRL1 antibodies capable of binding to TLNKFI. The anti-PRL antibody may have the ability to simultaneously bind to these two sequences.
[0079] Furthermore, Example 27 shows that the anti-PRL3 antibody can bind to the epitopes KAKFYN and HTHKTR. Therefore, the present inventors provide an anti-PRL antibody such as an anti-PRL3 antibody capable of binding to the sequence KAKFYN. The present inventors also provide anti-PRL antibodies such as anti-PRL3 antibodies capable of binding to HTHKTR. The anti-PRL antibody may have the ability to simultaneously bind to these two sequences.
[0080] The anti-PRL antibody may comprise the variable region of antibody 269, the variable region of antibod...
Embodiment 9
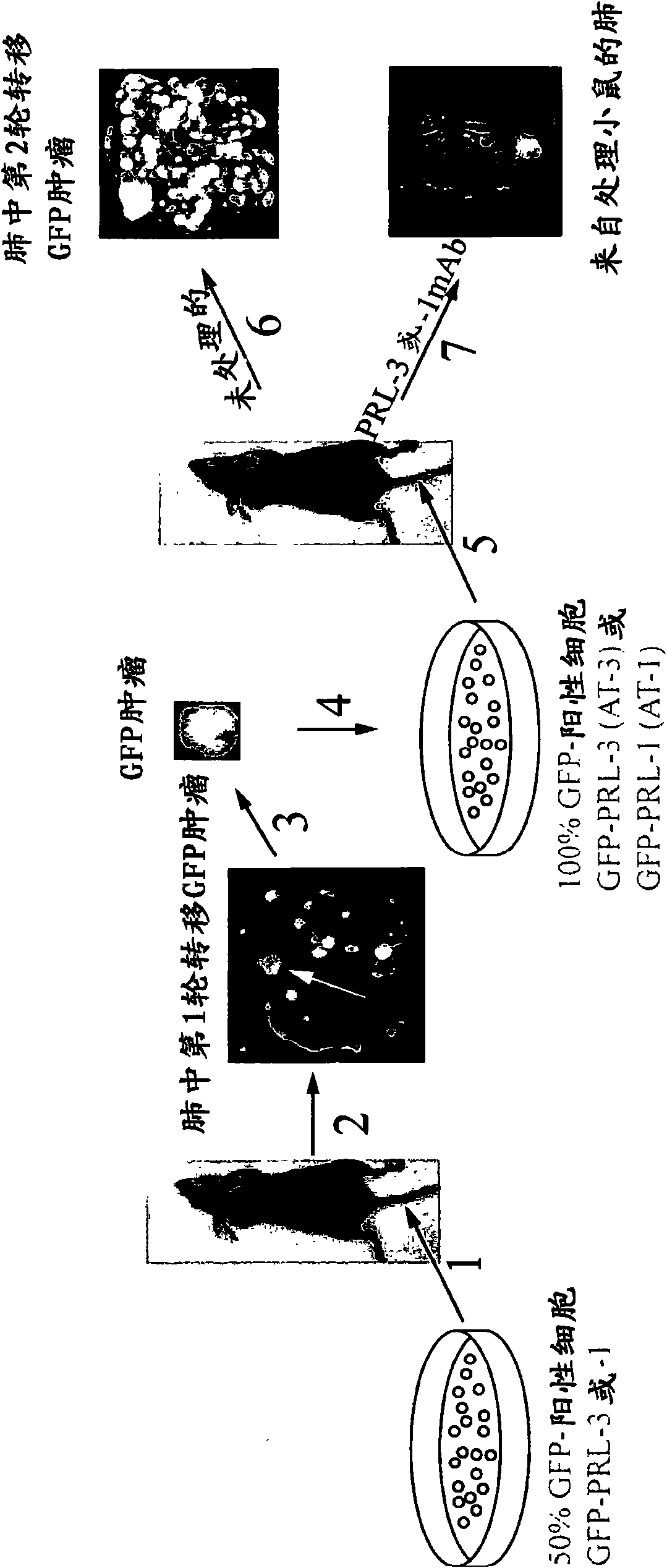
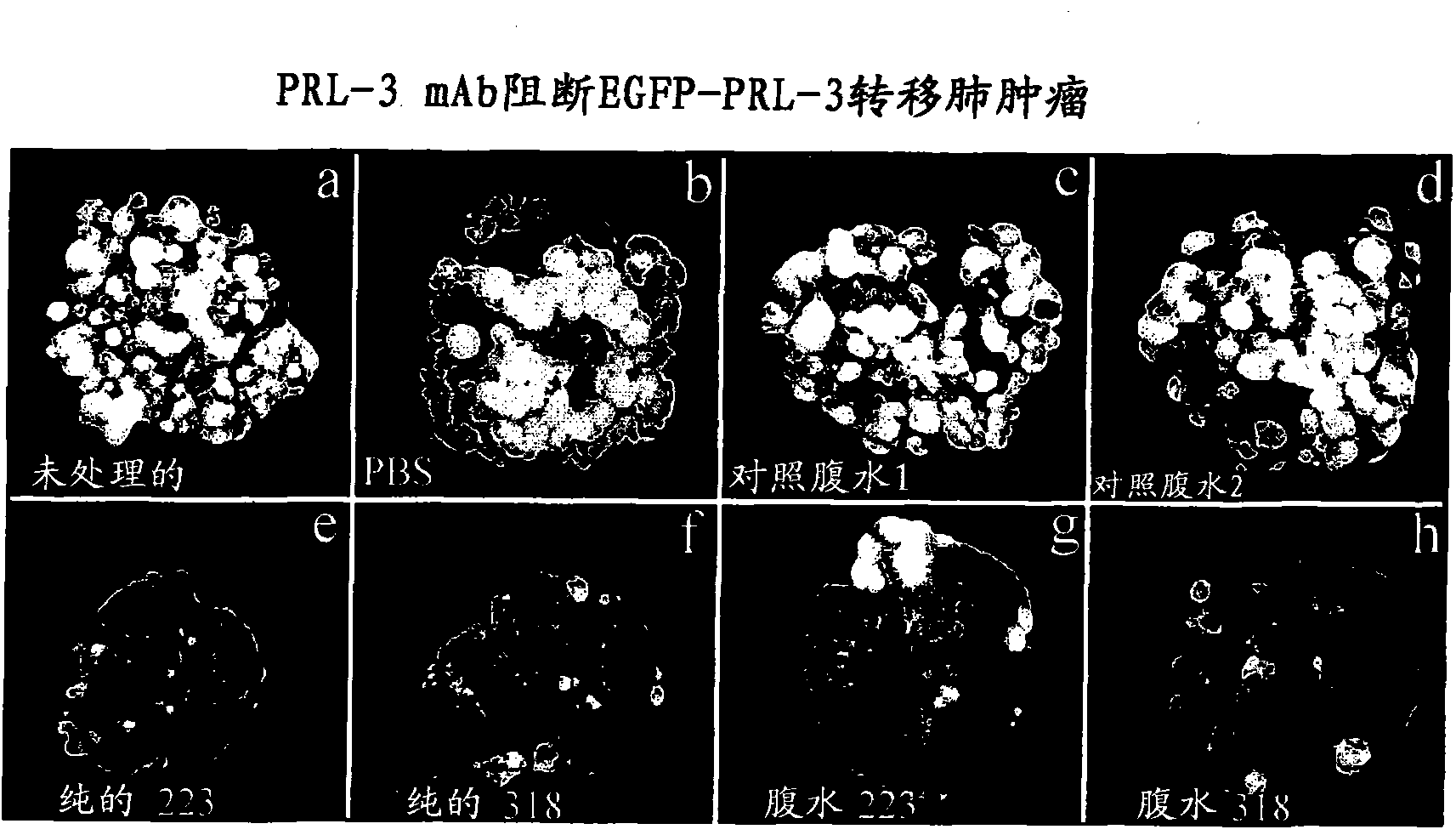
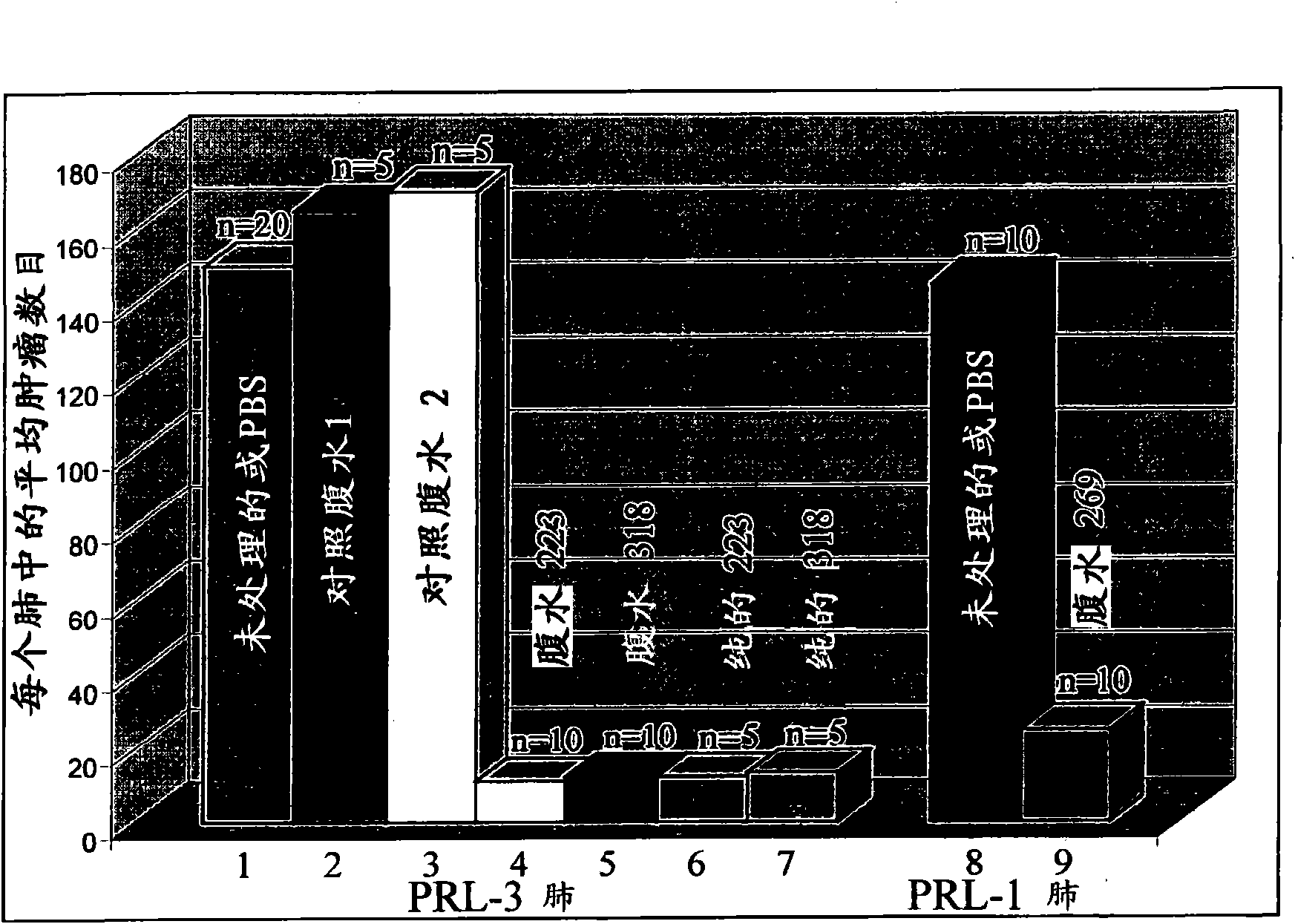
[0096] Example 9 records the generation of PRL overexpressing tumors in mice, and provides an animal model for metastasis and cancer treatment. Examples 10-12 show that animals treated with anti-PRL antibodies show significantly lower metastatic lung tumors compared to animals not treated with anti-PRL antibodies. Specifically, the treated animals showed about 90% fewer tumors than untreated animals. The anti-PRL antibody can bind to the PRL polypeptide and block its activity, even though the PRL polypeptide is localized in the cell. The inventor's research represents the first examples of how effective (about 90%) of metastasis can be blocked by using monoclonal antibodies against the respective phosphatases (although they are localized in the cell).
[0097] The inventors also showed that the anti-PRL-3 monoclonal antibody can effectively block the formation of metastases in the human ovarian cancer cell line A2780 expressing endogenous PRL-3 protein.
[0098] Therefore, the pr...
Embodiment 1
[0497] Example 1. Cell lines: CHO-K1, A2780 and CT26
[0498] CHO-K1 cells, A2780 human ovarian cancer cells and CT26 mouse colon cancer cells were purchased from ATCC (Manassas, VA).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com