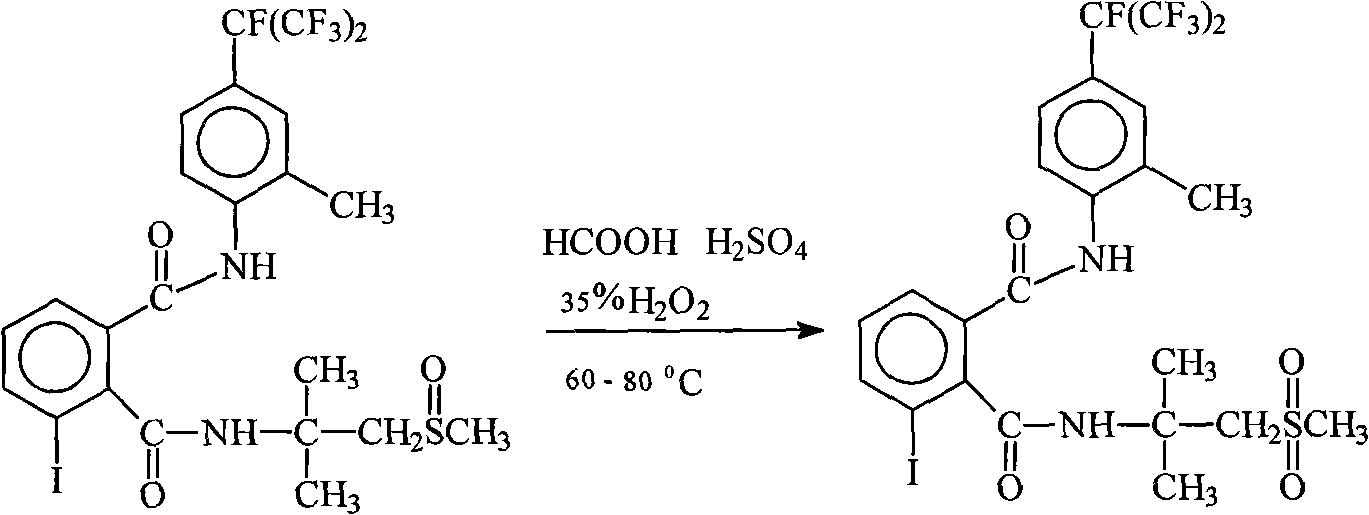
Method for preparing flubendiamide
A technology of fluraniliprole and phthalamide, which is applied in the field of preparation of flubendiamide, can solve the problems of non-recyclable solvent, excessive waste acid, excessive waste acid, etc., and achieves low cost, reduction of waste acid, simple and safe operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 450 milliliters of DMF, 50 grams of concentration by weight are 95% nitric acid, 2 grams of manganese acetate and 66.6 grams (0.1 mole) of N2-[1,1-dimethyl-2-methanylethyl]-3-I- Add N1-[2-methyl-4-(1,2,2,2-tetrafluoro-1-trifluoromethylethyl)phenyl]-1,2-benzenedicarboxamide into a 1000ml reaction flask , stirred and heated to 70° C., and air was introduced to carry out air oxidation. The reaction time was 8 hours, and the conversion of raw materials was greater than 99% by HPLC tracking. Recover DMF and nitric acid under reduced pressure, then add methanol and water for recrystallization. After drying, 60 g of product were obtained, a yield of about 88%.
Embodiment 2
[0018] 450 milliliters of DMF, 50 grams of concentration by weight are 95% nitric acid, 5 grams of manganese acetate and 66.6 grams (0.1 moles) of N2-[1,1-dimethyl-2-methylsulfonyl ethyl]-3-I -N1-[2-methyl 4-(1,2,2,2-tetrafluoro-1-trifluoromethylethyl)phenyl]-1,2-benzenedicarboxamide was added to a 1000 ml reaction flask , stirred and heated to 105° C., and air was introduced for air oxidation. The reaction time was 8 hours, and the conversion of raw materials was greater than 99% by HPLC tracking. Recover DMF and nitric acid under reduced pressure, then add methanol and water for recrystallization. After drying, 57 g of product were obtained, a yield of about 84%.
Embodiment 3
[0020] 400 milliliters of DMAC, 100 grams of 95% nitric acid, 2 grams of manganese acetate and 66.6 grams (0.1 moles) of N2-[1,1-dimethyl-2-methylsulfonylethyl]-3-I- Add N1-[2-methyl-4-(1,2,2,2-tetrafluoro-1-trifluoromethylethyl)phenyl]-1,2-benzenedicarboxamide into a 1000ml reaction flask , stirred and heated to 90° C., air was introduced to carry out air oxidation, the reaction time was 5 hours, and the conversion of raw materials was greater than 99% by HPLC tracking. Recover DMAC and nitric acid under reduced pressure, then add methanol and water for recrystallization. After drying, 56 g of product were obtained, a yield of about 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com