Alpha 7 nicotinic agonists and antipsychotics
A technology of antipsychotics and agonists, which is especially used in the application of mental disorders, mental disorders, and the treatment of psychiatric disorders, and can solve problems such as limiting the therapeutic index of nicotinic ligands.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0449] By combining (2S,3R)-N-(2-((3-pyridyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl)benzo furan-2-carboxamide and clozapine to prepare a pharmaceutical composition. The compositions contain respective amounts of (2S, 3R)-N-(2-((3-pyridyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl)benzofuran-2 - formamide and clozapine, delivering a therapeutically effective amount of each component on a daily basis. The composition is administered to a patient on a once-a-day, twice-a-day, three-times-a-day, or four-times-a-day basis for the treatment of schizophrenia.
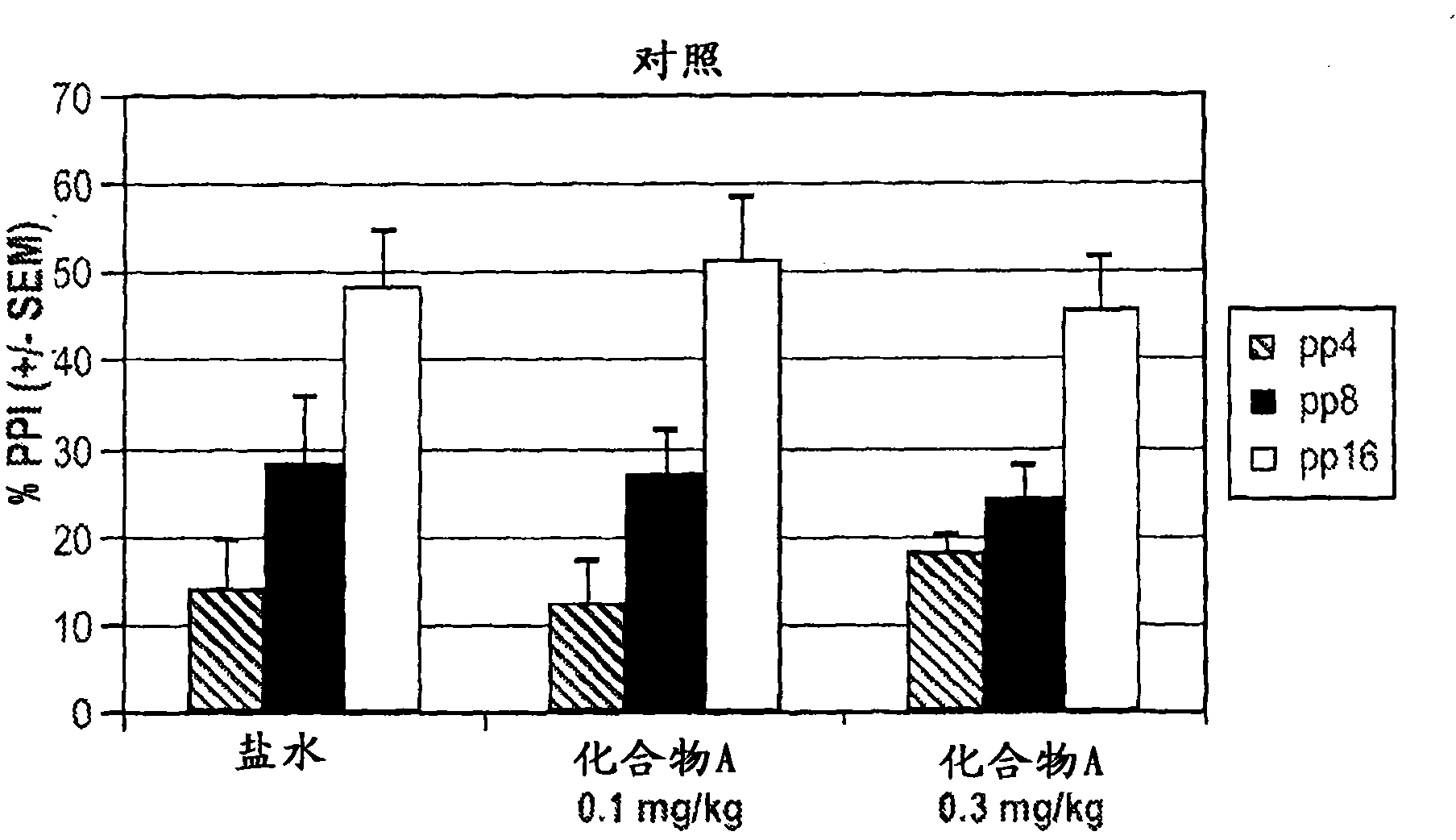
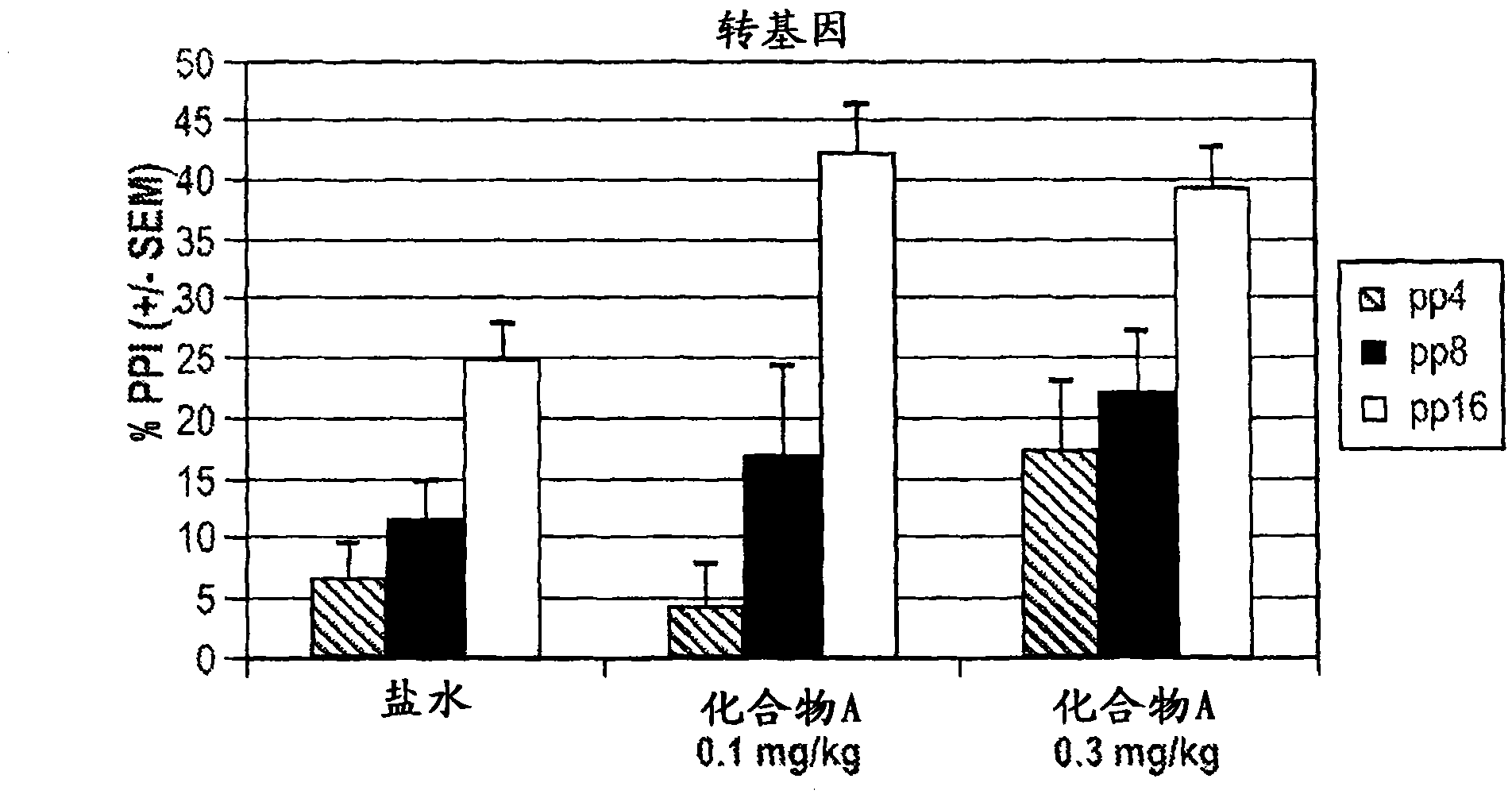
[0450] Clozapine (3.0 mg / kg) and Compound A (0.1 mg / kg) had little effect on PPI or panic when administered alone. see Figure 4 , 2a, and 2b. In contrast, there was a significant main effect (p=0.006) for the combination of clozapine (3.0 mg / kg) and Compound A (0.1 mg / kg) for the transgenic mice.
[0451] No synergy was observed in control wild-type mice. see Figure 5 and 6 .
Embodiment 2
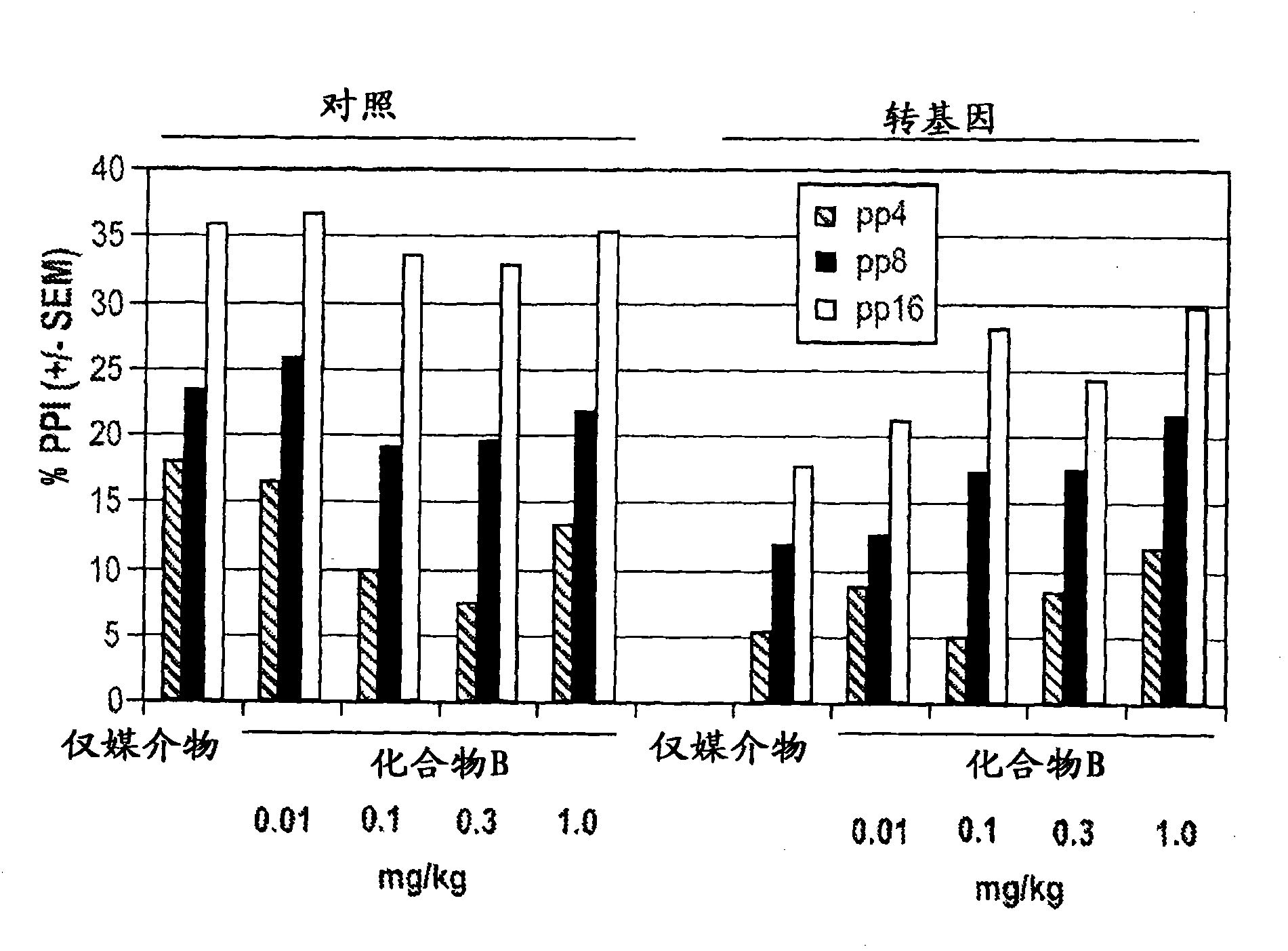
[0453] By combining (2S,3R)-N-(2-((3-pyridyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl)benzo furan-2-carboxamide and quetiapine to prepare a pharmaceutical composition. The compositions contain respective amounts of (2S, 3R)-N-(2-((3-pyridyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl)benzofuran-2 - formamide and quetiapine, delivering a therapeutically effective amount of each component on a daily basis. The composition is administered to a patient on a once-a-day, twice-a-day, three-times-a-day, or four-times-a-day basis for the treatment of schizophrenia.
[0454] In transgenic th(tk-) / th(tk-) mice, 3.0 mg / kg of quetiapine ( Figure 7a and 7b ) or 0.1mg / kg of Compound A ( Figure 2a and 2b ) independently has no significant main effect. However, when used in combination, there was a synergistic therapeutic effect (p=0.047) (Figure 8). [alpha]7 nicotinic agonists and antipsychotics work synergistically to increase prepulse inhibition in transgenic mice, th(tk-) / th(tk-) mice. In co...
Embodiment 3
[0460] binding assay
[0461] [ 3 H]-Methyl Nitanine ([ 3 H]-MLA) binding. [ 3 H]-Nicotine binds α4β2. NNRs were determined in rat cortical membrane preparations or SH-EP1 cells using standard methods modified from published methods (Lippiello and Fernandes, 1986). IC was determined by least squares nonlinear regression using GraphPad Prism software (GraphPAD, San Diego, CA). 50 (Concentration of compound producing 50% inhibition of binding). Using the Cheng-Prusoff equation (Cheng and Prusoff, 1973), calculate K i .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com