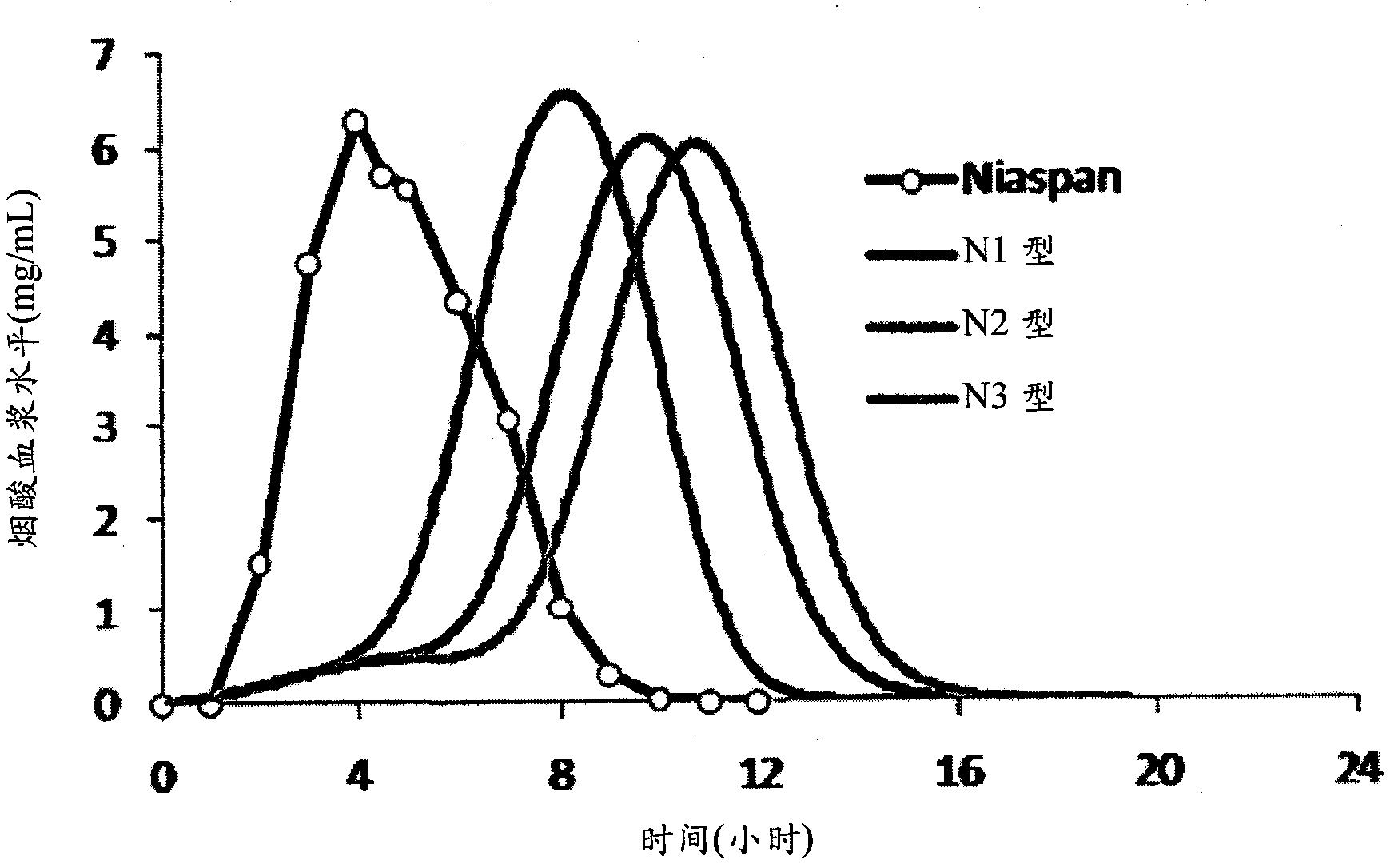
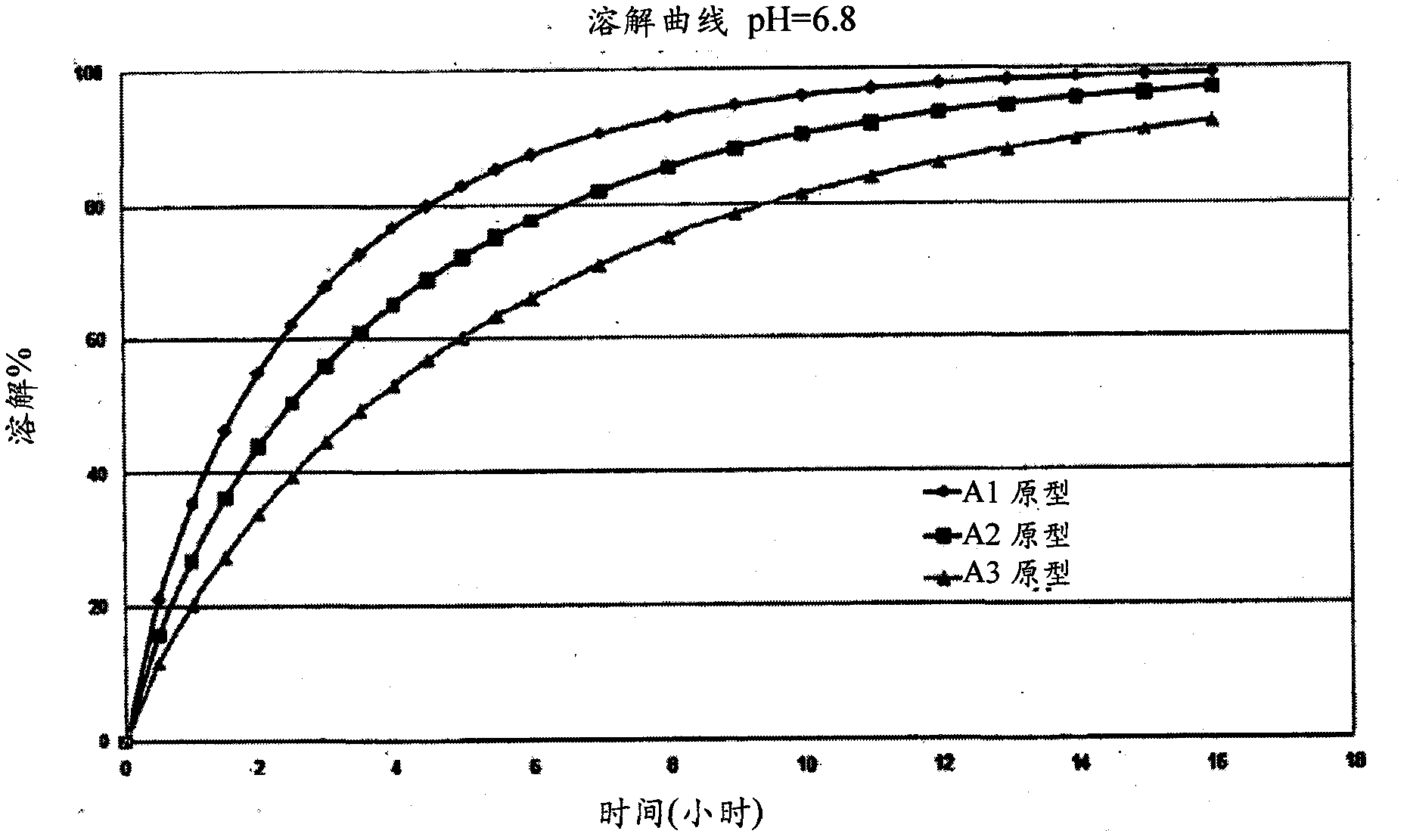
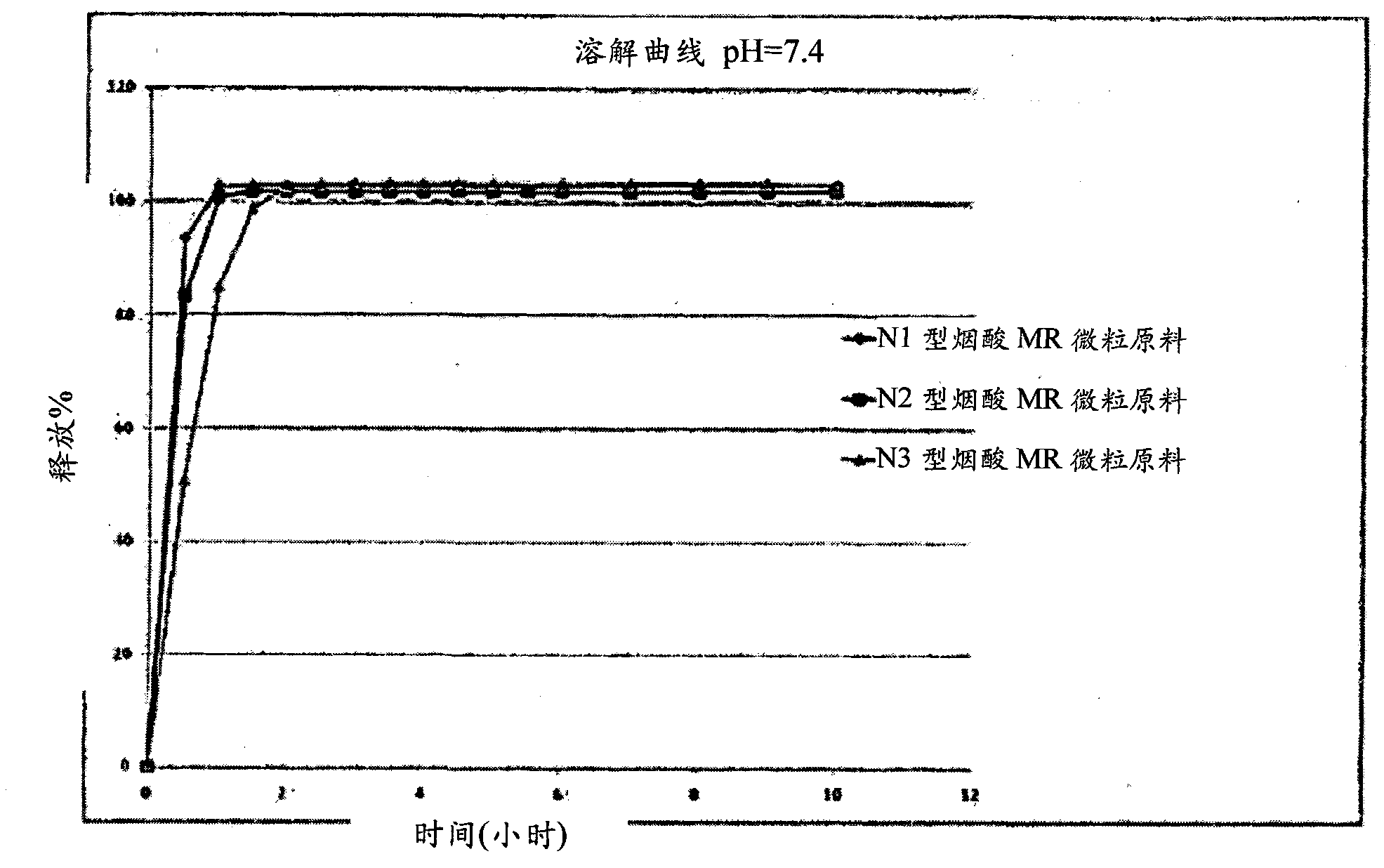
Niacin and NSAID combination therapy
A technology of niacin and therapy, which is applied in drug combinations, medical preparations containing active ingredients, metabolic diseases, etc., and can solve problems such as lowering and increasing expected tolerance and safety, and limiting doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0387] A clinical study is provided to establish the antiflush effect of an aspirin regimen on immediate-release niacin.
[0388] The product used is a niacin / aspirin oral dosage form.
[0389] Test design
[0390] The primary endpoint of the study was to compare the incidence, duration and severity of flushing after administration of niacin between treatment groups; adverse events were routinely monitored.
[0391] Subjects were screened once up to 4 weeks prior to the first dispensing visit. All eligible subjects received two single doses of niacin each week. Subjects were randomly assigned to receive either an aspirin regimen or a placebo regimen on Day 1 prior to their niacin dose and the opposite regimen prior to their niacin dose on Day 8. Subjects were randomly selected for blind testing on the morning and evening of Days -4, -3, -2, and -1. Subject was admitted to the hospital on Day -1 and remained under observation for approximately 24 hours after administration ...
Embodiment 2
[0441] Research
[0442] A randomized, double-blind, three-way cross-over study was provided to investigate the antiflush effect of two aspirin regimens compared with placebo on extended-release niacin.
[0443] product
[0444] The product used is a niacin / aspirin oral dosage form.
[0445] Research purposes
[0446] Evaluation of two aspirin regimens for relief versus single-dose administration of extended-release niacin (Niaspan ) related flushing abilities.
[0447] method
[0448] Extended-release niacin (2 g) and one of two aspirin regimens or placebo were administered as a single oral dose. Subjects remained overnight at the Clinical Research Center following niacin administration to carefully monitor for flushing reactions and other adverse events. Each subject received all three dosage regimens in a randomized, three-way spanning fashion.
[0449] primary endpoint
[0450] The incidence, duration, and severity of flushing after niacin administration were comp...
Embodiment 3
[0535] Aspirin extended-release and niacin-modified-release have been manufactured at the French company FT (Flamel Technologies) by the methods described in US 5,846,566, US 5,603,957 and WO 03 / 030878.
[0536] Aspirin SR (ASA) is an extended release product in the form of white / white oral capsules containing 81 mg of aspirin as aspirin microparticles. ASA is available in three extended release formulations that release 80% of aspirin in approximately 4-5 hours (prototype A1), 6-7 hours (prototype A2) and 9-10 hours (prototype A3) SR capsules. The A1, A2 and A3 prototypes were made with the same coating composition with different coating ratios. The coating composition is not pH sensitive and its dissolution is not affected by its location within the gastrointestinal tract.
[0537] The manufacturing method of Aspirin SR is based on the coating of aspirin crystals of suitable shape and size as 300 / 500 grade refined acetylsalicylic acid supplied by Shandong. The quantitativ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com