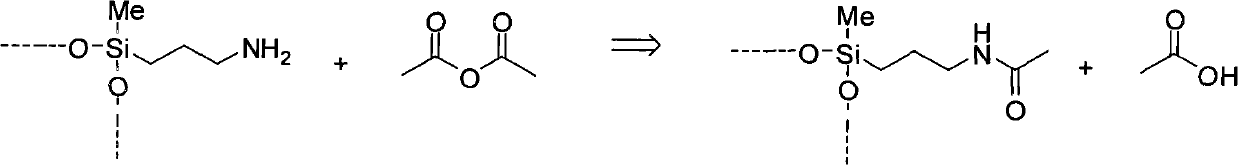
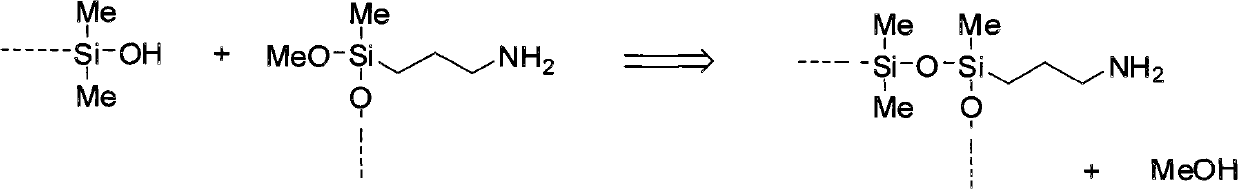Process for the preparation of amine-amide-functional siloxanes
An amino-functional, siloxane-based technology, applied in chemical instruments and methods, improved hand fibers, and compounds of elements in group 4/14 of the periodic table, can solve problems such as increased viscosity and affecting application properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Mix 191 g of the general formula [HOSiMe 2 o 1 / 2 ] 2 [SiMe 2 o 2 / 2 ] 38 of siloxane and 7.4g of formula Me(EtO) 2 Si(CH 2 ) 3 NH 2 aminofunctional silane, and added 1.3 g of acetic anhydride. The mixture was heated to 90 °C and stirred at 100 mbar for 3 h. The distillate formed was removed from the reaction mixture. This gives an amido-amino-functional siloxane with a viscosity of 4500 mPas.
Embodiment 2
[0132] In a 250 ml three-necked flask equipped with a precision ground glass stirrer and thermometer, 189 g of the general formula [HOSiMe 2 o 1 / 2 ] 2 [SiMe 2 o 2 / 2 ] 38 Siloxane, 7.3g formula Me(EtO) 2 Si(CH 2 ) 3 NH 2 Aminofunctional silane and 3.0 g of trimethylethoxysilane, and 1.3 g of acetic anhydride was added. The mixture was heated to 90 °C and stirred at 100 mbar for 3 h. The distillate formed was removed from the reaction mixture. An amido-amino-functional siloxane with a viscosity of 2600 mPa s was obtained. 29 Si-NMR spectroscopic studies showed that 53% of the chain end groups of the obtained polymer were trimethylsilyl groups.
Embodiment 3
[0134] In a 250 ml three-necked flask equipped with a precision ground glass stirrer and thermometer, 189 g of the general formula [HOSiMe 2 o 1 / 2 ] 2 [SiMe 2 o 2 / 2 ] 38 Siloxane, 7.3g formula Me(EtO) 2 Si(CH 2 ) 3 NH 2 Aminofunctional silane and 2.0 g of bis(trimethylsilyl)amine, and 1.3 g of acetic anhydride was added. The mixture was heated to 90 °C and stirred at 100 mbar for 3 h. The distillate formed was removed from the reaction mixture. An amido-amino-functional siloxane with a viscosity of 2100 mPa s was obtained. 29 Si-NMR spectroscopic studies showed that 61% of the chain end groups of the obtained polymer were trimethylsilyl groups.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap