Non-typhoidal salmonella vaccines
A Salmonella and vaccine technology, applied in the direction of antibacterial drugs, bacterial antigen components, depsipeptides, etc., can solve problems such as weakening of binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Materials and methods
[0061] Mice, Bacterial Strains and Immunogens
[0062] Wild-type (WT) mice were obtained from domestic populations. The origin of genetically deficient mice has been reported elsewhere (Cunningham et al. (2007) J. Immunol. (Journal of Immunology) 178 , 6200-7 and Gaspal et al., (2008) J. Immunol. (Journal of Immunology) 180 , 2824-9), with the exception being the βδ TCR-deficient mouse, which was obtained from Jax. All mice and groups were age (6-12 weeks) and sex matched prior to use. Salmonella enterica typhimurium serotype SL1344 is a WT strain, and SL3261 is a well-described AroA-deficient attenuated strain. OmpD-deficient STm was generated on the SL3261 background.
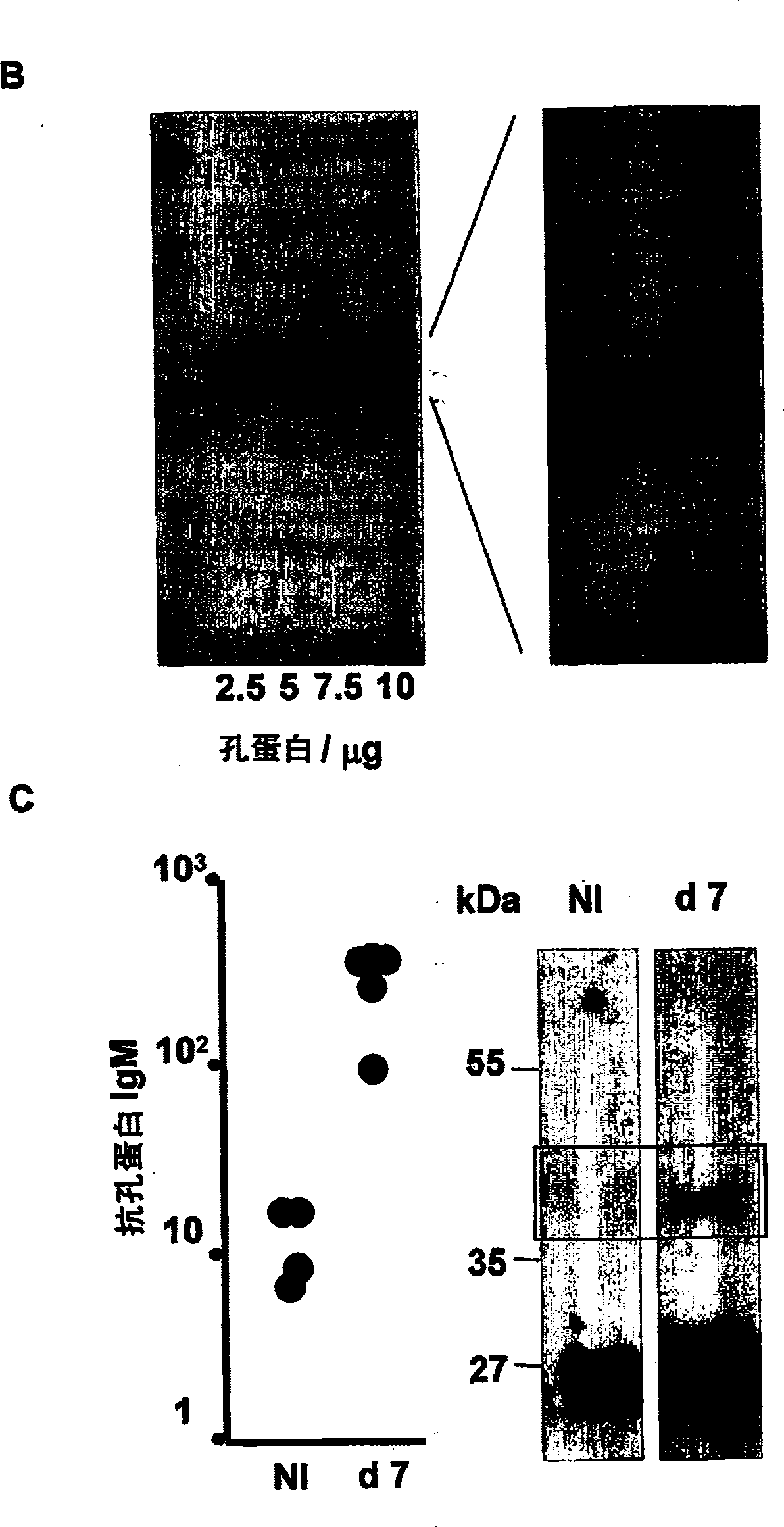
[0063] Total Omp preparations were generated by extracting cell envelopes with 2% (v / v) Triton X-100 and harvesting by centrifugation as previously described. Purified porins were produced from ST (strain 9933) and STm (ATCC strain 14028) using well-established methods...
Embodiment 2
[0108] When appropriate, the following further work was carried out following the protocol in Example 1:
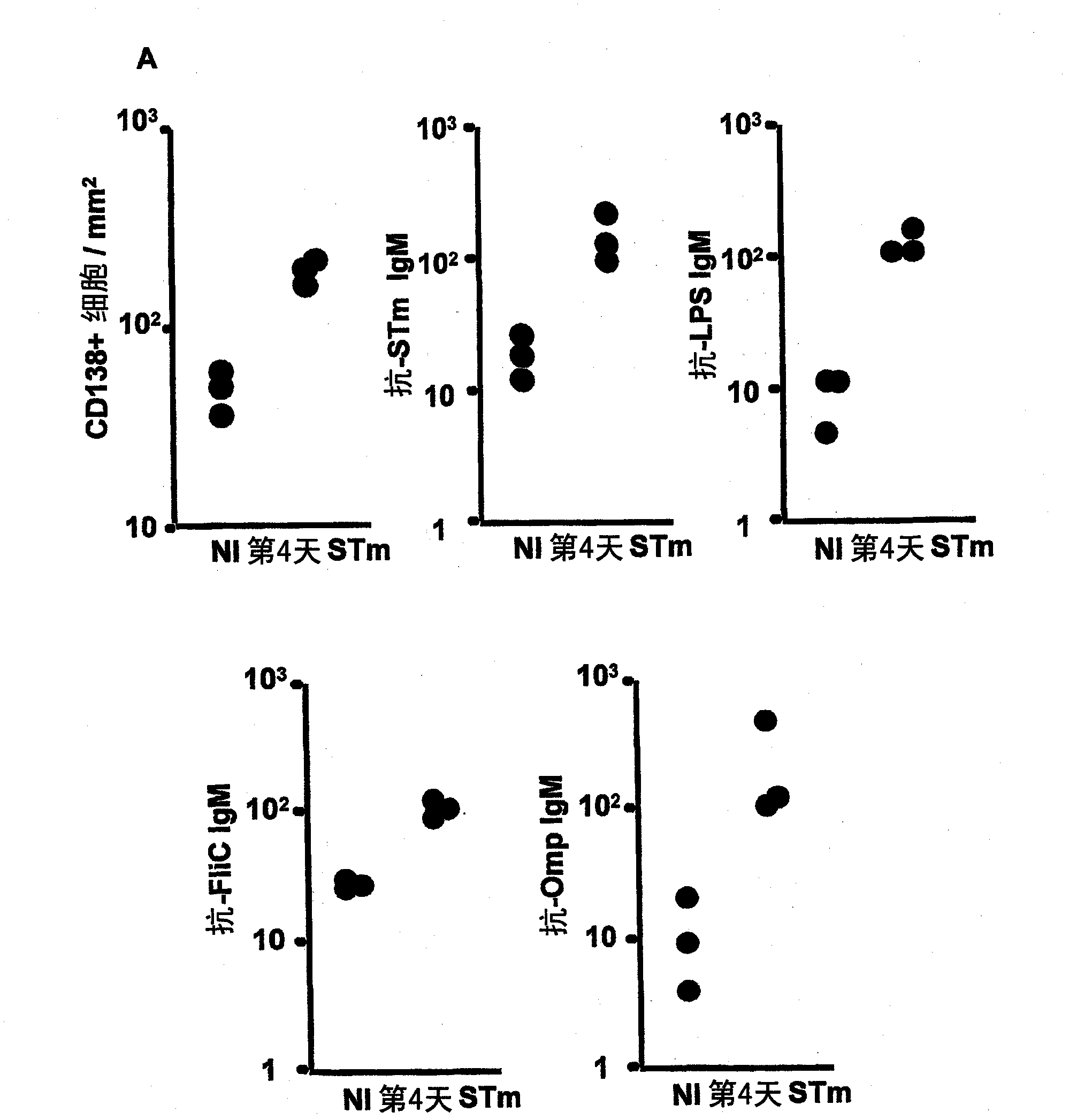
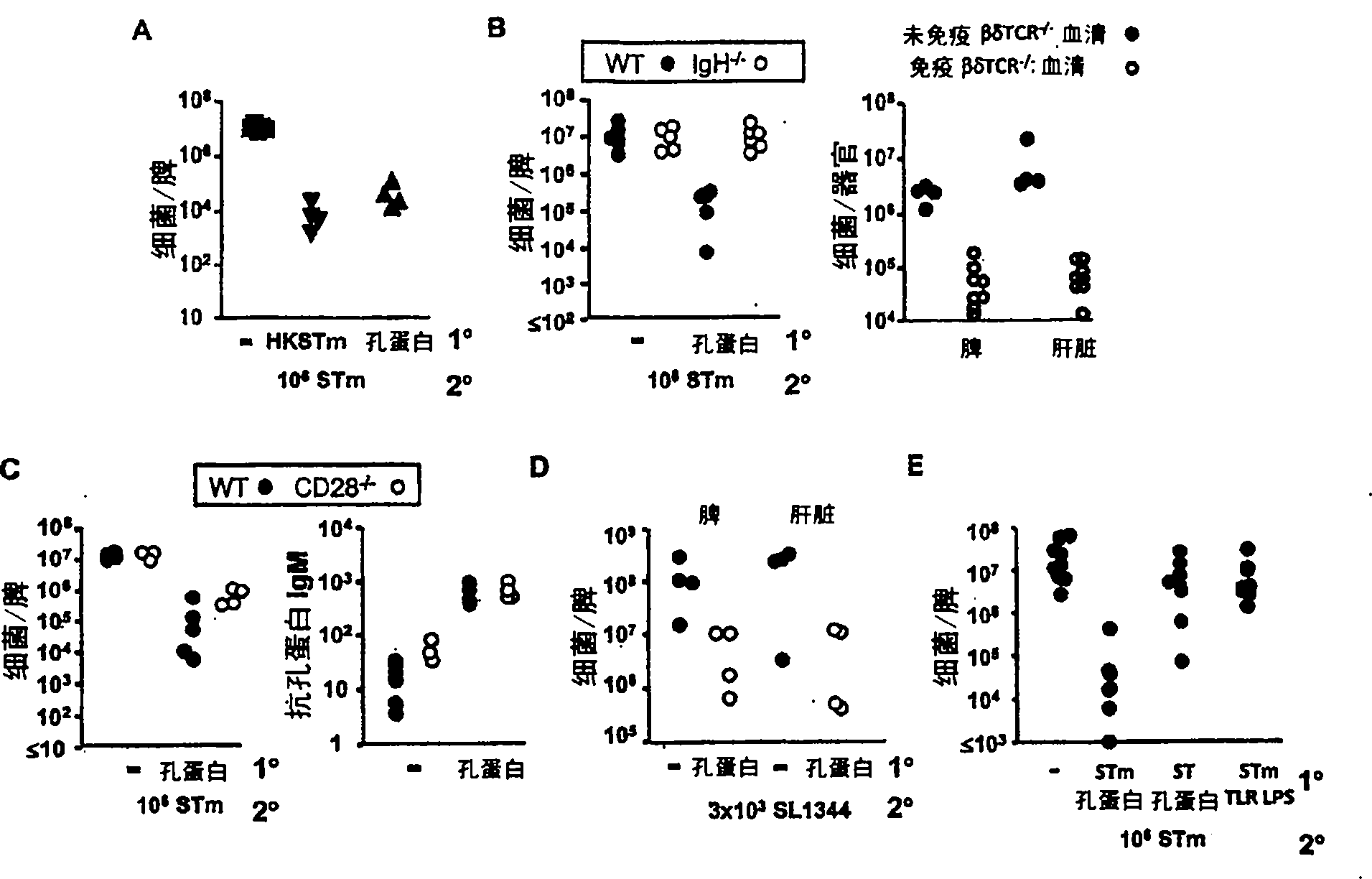
[0109] 1. In NTS, bacteremia often reflects disease severity. We therefore investigated the effect of porin immunization on bacteremia induced by subsequent infection with STm or STm lacking OmpR (resulting in defective OmpF and OmpC expression) or OmpD deficient STm. We show that porin immunization can eliminate bacteremia in STm infection ( Figure 5 , left panel) and can be seen after infection of porin-primed mice with OmpR-deficient STm ( Figure 5 , central panel), but rarely seen after infection with OmpD-deficient STm ( Figure 5 , right panel). This suggests that antibodies against porins, especially OmpD, can significantly reduce bacteremia.
[0110] 2. A single immunization with porins is sufficient to reduce the bacterial burden caused by STm. To assess whether two immunizations increased the beneficial effects of porin immunization, mice were immunized t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com