SiRNA for inhibiting gene expression of caspase-3
A gene expression and sequence technology, applied in the field of siRNA, can solve the problem of not having the most suitable siRNA, and achieve the effect of reducing apoptosis and necrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Synthesis of siRNA inhibiting caspase-3 gene expression.
[0024] An siRNA sequence that inhibits the expression of caspase-3 gene is formulated, and a random sequence is selected as a negative control. A, G, C, U in caspase-3 siRNA sequence represent adenine ribonucleotide, guanine ribonucleotide, cytosine ribonucleotide and uracil ribonucleotide, T represents thymine deoxyribonucleotide glycosides.
[0025] The synthetic siRNA sequence is as follows:
[0026] Sense strand: 5'-GAGTCTGACTGGAAAGCCGAA-3'
[0027] Antisense strand: 5'-TTCGGCTTTCCAGGAAGACTC-3'.
Embodiment 2
[0028] Example 2: Primary culture of nerve cells.
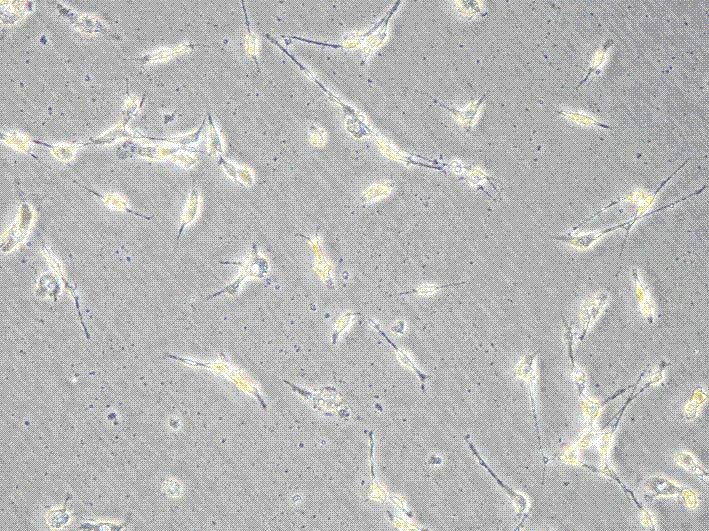
[0029] Select newborn SD mice of 1-3 days, soak them in 75% ice alcohol for 2 minutes, take out the brain under aseptic conditions, remove the meninges and white matter, collect the cerebral cortex and make a homogenate, put it in 5mL In a centrifuge tube with 0.25% (w / v) trypsin preheated at 37°C, pipette gently to make a single cell suspension. Stand still, transfer the upper cell suspension into a centrifuge tube containing 2mL whole cell culture medium to stop digestion, filter with a 220nm filter, centrifuge at 1000r / min for 10min, discard the supernatant, add serum-free cell culture medium, pipette evenly, 1000r / min Centrifuge for 5 minutes, discard the supernatant, add the whole cell culture medium, pipette evenly, and adjust the cell concentration to 1×10 with a cell counting plate. 5 cells / mL, inoculated on culture flasks or dishes previously covered with poly-lysine, at 37°C, 5% CO 2 After culture, 24 hours after ...
Embodiment 3
[0030] Example 3: Infection of nerve cells.
[0031] Make the concentration 1×10 5Nerve cell suspension per mL was inoculated in a 96-well plate, and 100 μL of culture medium was added to each well. On the 4th day of cell culture, select the same batch of well-growing nerve cells and divide them into normal living cell group, 1mM aluminum-stained group, 1mM aluminum-stained + caspase-3 RNAi virus group, and observe the growth status and morphological changes of the cells . The specific conditions are as follows: only 90 μL of culture medium was added to the normal living cell group; 90 μL of culture medium containing aluminum was added to the 1 mM aluminum-stained group until the final concentration of aluminum was 1 mM; 90 μL of culture medium containing aluminum was added to the 1 mM aluminum-stained + caspase-3 RNAi virus group The virus vector and caspase-3 siRNA interference reagent were added at the same time until the final concentration of aluminum was 1 mM. Put the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com