High-transduction-efficiency hepatophilic cell hepatitis C virus pseudo-particle and envelope protein coded sequence thereof
A hepatitis C and liver cell technology, applied in the field of pseudovirion particles and its preparation, can solve the problems of low virus yield and transduction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Cloning of HCV envelope protein full-length gene
[0021] 1. Extraction of HCV nucleic acid
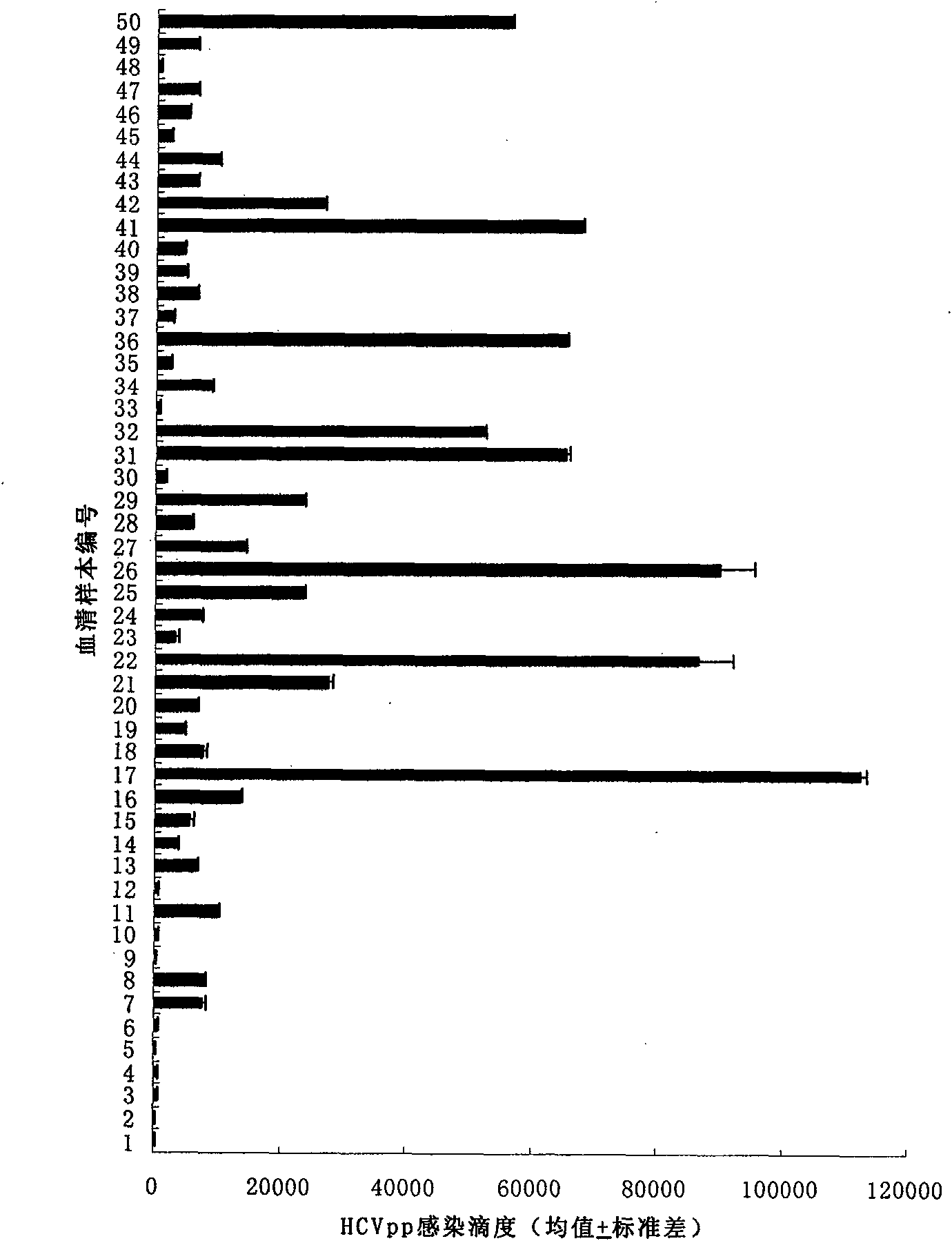
[0022] Viral nucleic acid RNA was extracted from the sera of 50 HCV RNA-positive patients, respectively, using the QIAamp Ultraseus virus kit from QIAGEN Company.
[0023] Specific method: Take 1ml of serum to a 2ml EP tube and equilibrate the temperature to 15°C-25°C, add 800ul Buffer AC, add 5.6ulcarrier RNA to the lid of the EP tube, then vortex for 10s to mix; incubate at room temperature for 10 minutes, then centrifuge at 3200rpm for 3min , discard all the supernatant; add 300ul Buffer AR (preheated at 60°C) and 20ul proteinase K, and vortex to dissolve the precipitate; vortex once for 5 seconds during 10 minutes in a water bath at 40°C, and transfer it all into the QIAamp Spin Column 6000rpm Centrifuge for 1min to discard the waste liquid; add 500ul Buffer AW1 and centrifuge at 7500rpm for 1min to discard the waste liquid, add 500ul Buffer AW2 and centrifug...
Embodiment 2
[0040] Example 2: Screening of functional HCV envelope protein coding sequences
[0041] 1. Construction of HCV envelope protein expression plasmid
[0042] The correct HCV envelope protein gene identified by enzyme digestion and sequencing was amplified by PCR. The primer sequences were designed and synthesized according to the 5' and 3' end sequences obtained by sequencing, and the enzyme cutting site Nhe was added to the 5' and 3' ends respectively. I and EcoRI. The PCR amplified product was digested with NheI and EcoRI, inserted into the plasmid expression vector pCI-neo (promega product), identified by enzyme digestion and then sequenced. The correct plasmid was used to make HCVpp.
[0043] specific method:
[0044] A restriction endonuclease digestion: the identified correct clone with the HCV envelope protein coding sequence and the pCI-neo expression plasmid were digested with NheI and EcoRI restriction endonuclease respectively, and the enzyme digestion system was:...
Embodiment 3
[0054] Embodiment 3: Optimization of HCV envelope protein gene
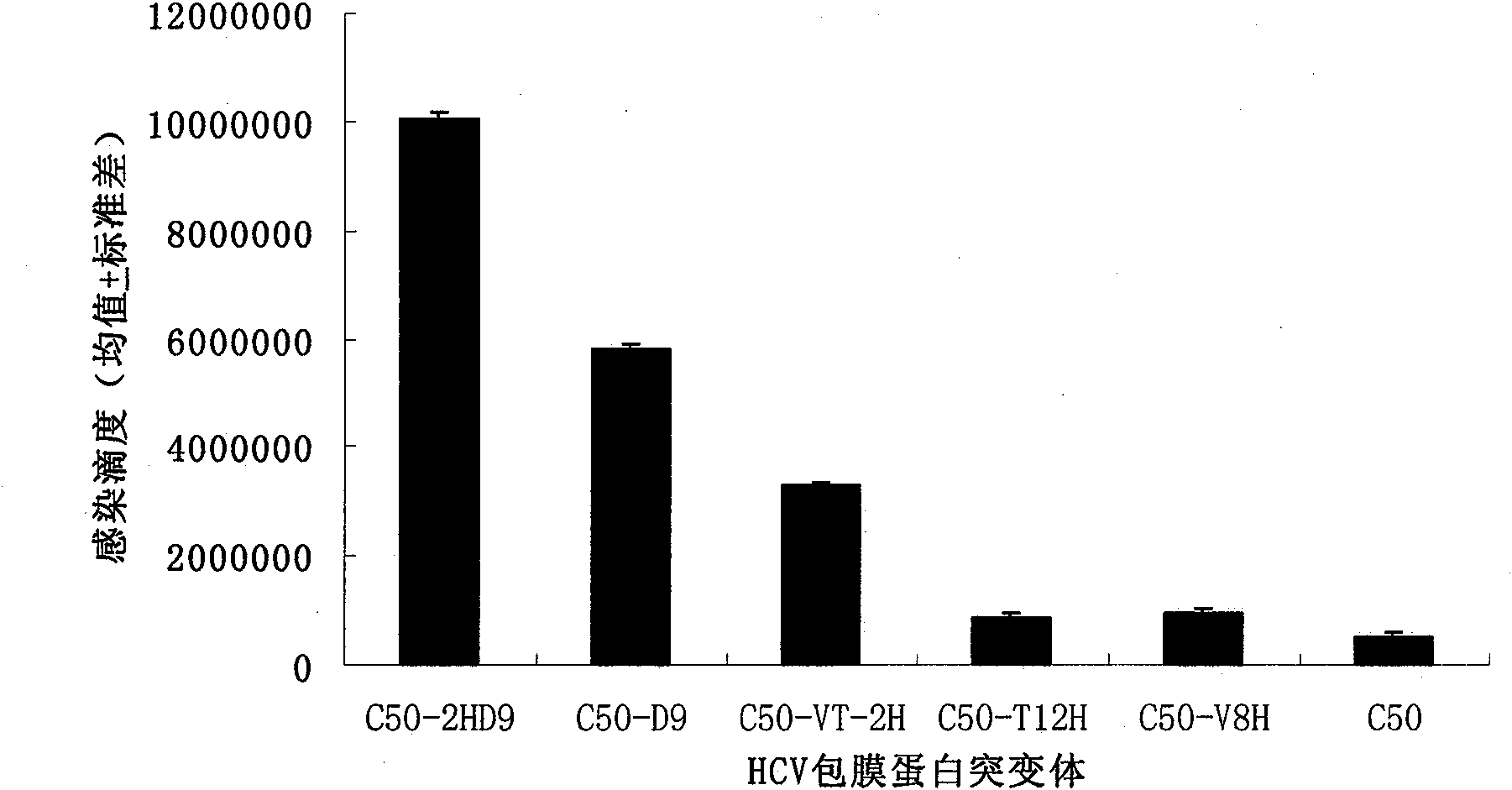
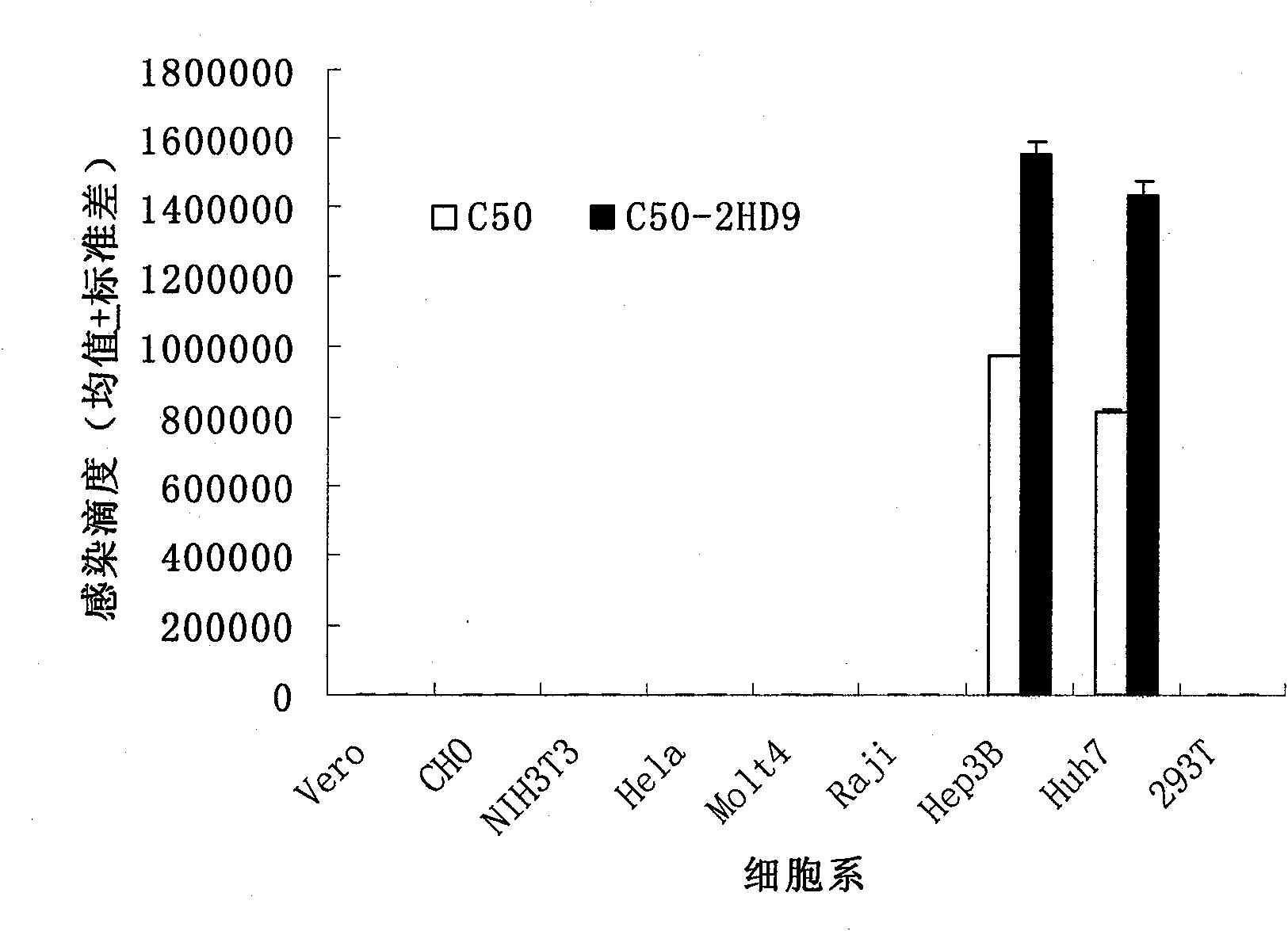
[0055] 1. Our previous studies have shown that the basic amino acid residues in the HCV envelope protein HVR1 have an important impact on the infectivity of HCVpp, especially if the 8th and 12th amino acid residues are basic amino acids, they can significantly enhance HCVpp infectivity. Based on these findings, we mutated the 8th valine residue (V) and the 12th threonine residue (T) of the C50 strain HCV envelope protein gene encoding HVR1 into histidine residues individually or simultaneously. Base, using Stratagene kit (QuikChange TM Site-Directed Mutagenesis Kit) for mutation. The mutants were named as C50-V8H, C50-T12H, and C50-VT-2H, respectively.
[0056] 2. Our previous studies also showed that amino acid residues 16-24 in the HCV envelope protein HVR1 sterically hinder the binding of the HCV envelope protein to the SR-BI receptor, and deletion of this peptide can significantly enhance the binding of H...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap