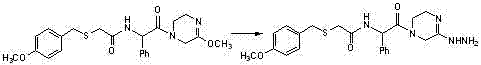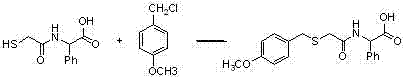Benzylthio acetamido acetylpyrazine triazole derivative as well as preparation thereof and application thereof
A technology of acetylpyrazinetriazole and benzylthioacetamide is applied in the field of benzylthioacetamido acetylpyrazinetriazole derivatives and preparation thereof, and can solve the problems of harsh production conditions, reduced cost, high cost and the like , to achieve the effect of easy operation, reasonable design and good production feasibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
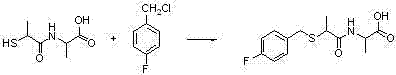
[0033] Weigh N-(2-mercaptopropionyl)-glycine (tiopronin) (3.92g, 0.024mol) and dissolve it in 20ml of N,N-dimethylformamide (DMF), then weigh potassium carbonate (8.28g , 0.06mol), stirred at room temperature for 15min; dropwise added p-methoxychlorbenzyl (3.76g, 0.024mol) into the single-necked bottle, stirred at room temperature for 3h, TLC detected that the reaction was complete; after the reaction was complete, adjust the pH with saturated aqueous sodium hydroxide solution value to 13, extracted 3 times with ethyl acetate (20ml / time), mainly to remove impurities; then adjusted the pH value to about 2 at low temperature, extracted 3 times with ethyl acetate (40ml / time), and combined the organic layers and dried, filtered, and evaporated to dryness under reduced pressure to obtain compound (b-1) with a yield of 82%.
[0034] The 1H NMR spectrum of the compound in deuterated chloroform: δ1.48(CH3,d,3H),3.65(CH,m,H),3.70(CH2,m,2H),3.72(CH3,m,3H) , 4.14 (CH2, s, 2H), 6.65 (2CH...
Embodiment 2
[0037]
[0038] Weigh compound (b-1) (0.85g, 0.003mol) and dissolve it in 10ml of DMF, then add 1ml of triethylamine, stir at room temperature for 10min; then add EDC (0.86g, 0.0045mol), HOBT (0.2g, 0.0015mol ) and 2-piperazinone (0.3g, 0.003mol), stirred at room temperature for 5h, TLC detected to complete reaction; evaporated DMF under reduced pressure, added saturated aqueous sodium bicarbonate solution, extracted with ethyl acetate, combined organic layer, dried and filtered , and evaporated to dryness under reduced pressure to obtain compound (c-1) with a yield of 85%.
[0039] The 1H NMR spectrum of the compound in deuterated chloroform: δ1.48(CH3,d,3H),3.46(2CH2,s,4H),3.65(CH,m,H),3.70(CH2,m,2H) , 3.72 (CH3, m, 3H), 4.09 (2CH2, s, 4H), 6.65 (2CH, d, 2H), 6.95 (2CH, d, 2H) ppm.
[0040] MS: m / z: 366.14 (M+1).
Embodiment 3
[0042]
[0043] Add trimethyloxonium tetrafluoroboric acid (207mg, 1.4mmol) to a solution of compound (c-1) (438.54mg, 1.2mmol) in dichloromethane (6ml), stir at room temperature overnight, and mix the reaction solution with bicarbonate Sodium aqueous solution was mixed, extracted twice with ethyl acetate, the organic phase was washed with brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain compound (d-1) with a yield of 86%.
[0044] The 1H NMR spectrum of the compound in deuterated chloroform: δ1.48(CH3,d,3H),1.6(CH2,t,2H),3.2(2CH2,t,4H),3.39(CH3,s,3H) ,3.65(CH,m,H),3.70(CH2,m,2H),3.72(CH3,m,3H),4.09(CH2,s,2H),6.65(2CH,d,2H),6.95(2CH, d,2H) ppm.
[0045] MS: m / z: 380.16 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com