Methods and compositions containing mTOR inhibitors for enhancing immune response
A technology of immune response and composition, applied in the field of regulating immune response, can solve problems such as difficulty in achieving successful application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] This example provides a description of the materials and methods used to obtain the data as described in Examples 2-9.
[0055] Mice and reagents: Housed, housed and used C57BL / 6, CD4 at RPCI according to IACUC guidelines + TCR transgenic Rag2 -1- (OT-II), CD8 + TCR transgenic Rag2 1 / - (OT-I, wild type), Stat4 -1- OT-I Rag2 -1- , and Tbx21 -1- OT-I Rag2 -1- mice. rmIL-12 (2ng / ml) was provided freely by Wyeth Ltd. (Cambridge, MA). IFN-a was freely provided by T. Tomasi (RPCI). rmlL-7 was purchased from Peprotech (Rocky Mount, NJ). 2-DG, 4-HT and rapamycin were purchased from Sigma-Aldrich (St. Louis, MO). LY290042 was purchased from Calbiochem. Insulin was purchased from Novo Nordisk (Princeton, NJ).
[0056] Stimulation of OT-I cells. According to known techniques, expressing H-2K with b / Ovalbumin antigen and B7.1 latex microspheres to stimulate native OT-I cells. Naive OT-II cells were stimulated with anti-CD3- / anti-CD28-coated latex beads. In some ex...
Embodiment 2
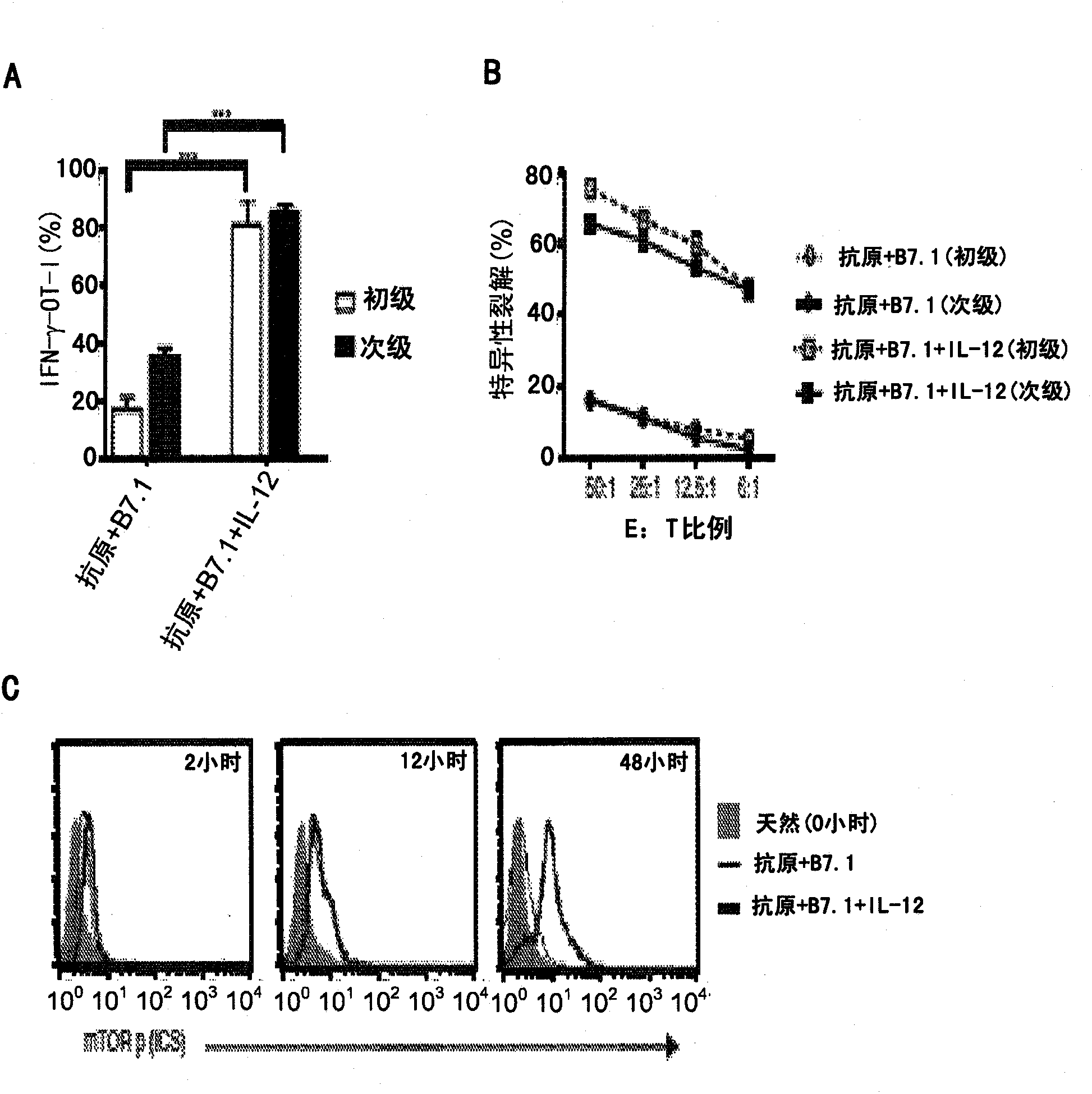
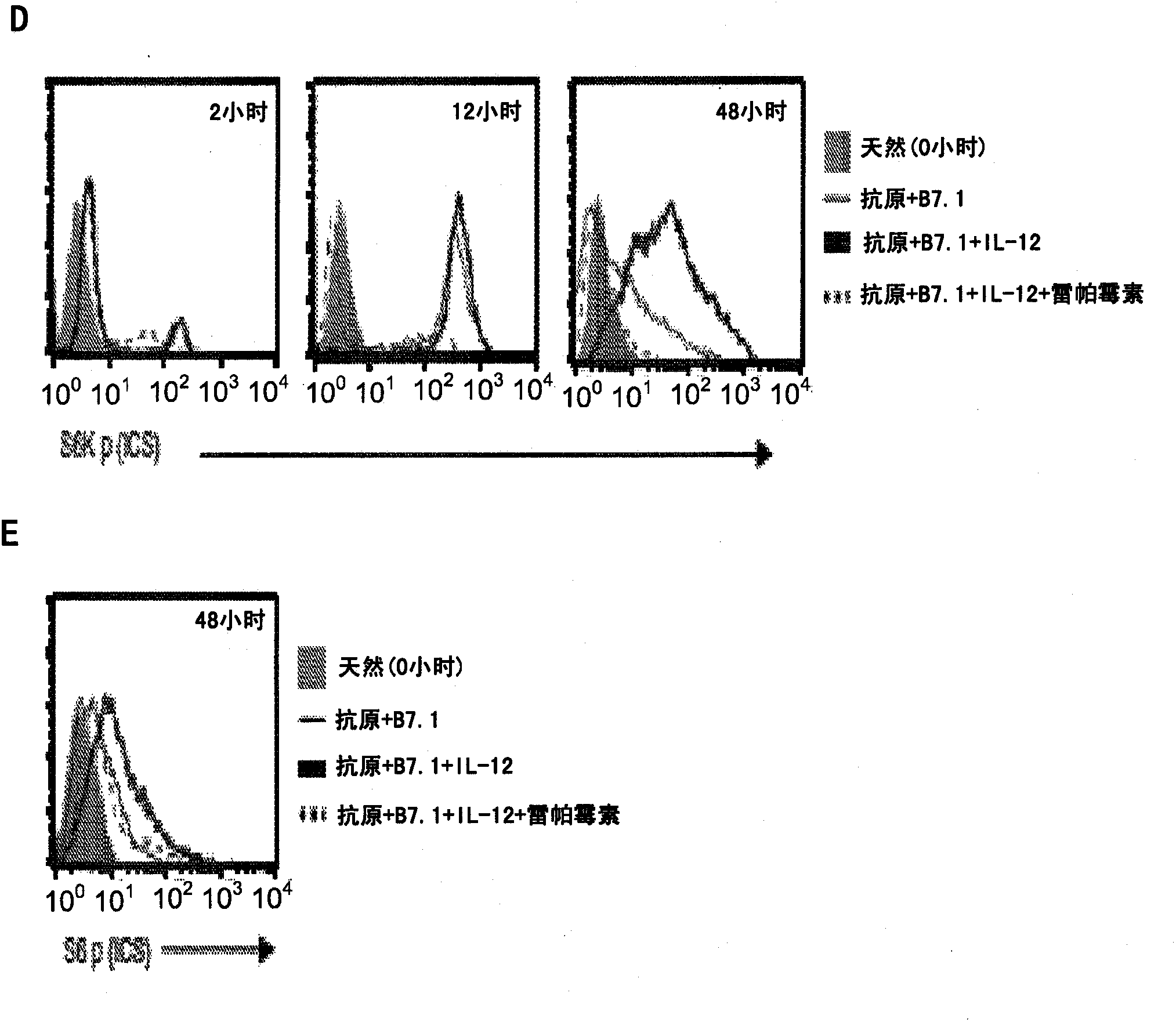
[0061] This example demonstrates that priming native CD8 + Instructions for T cells to undergo type I effector cell differentiation enhance mTOR activity. To study native CD8 + Mechanisms by which T cell instructive (signals 1, 2 and 3 - antigen [Ag], B7.1 [co-stimulatory] and IL-12 [cytokine]) programming into type I effector cell function we set out to confirm IL-12 plays a decisive role in the maturation of OT-I cells to type I effector cells stimulated with an adherent cell line called BOK expressing H-2K b , OVAp and B7.1. Addition of IL-12 resulted in massive production of IFN-γ and cytotoxic T lymphocyte (CTL) activity in OT-I cells at 72 hours ( figure 1 A and 1B, Elementary). In addition, when primary effector OT-I cells (72 hours) were placed in IL-7 for an additional 72 hours (12% IFN-γ measured at 144 hours) and restimulated with Ag and B7.1 (see Example 1) , only IL-12-conditioned OT-I cells re-induced IFN-γ and CTL activity ( figure 1 A and 1B; secondary). ...
Embodiment 3
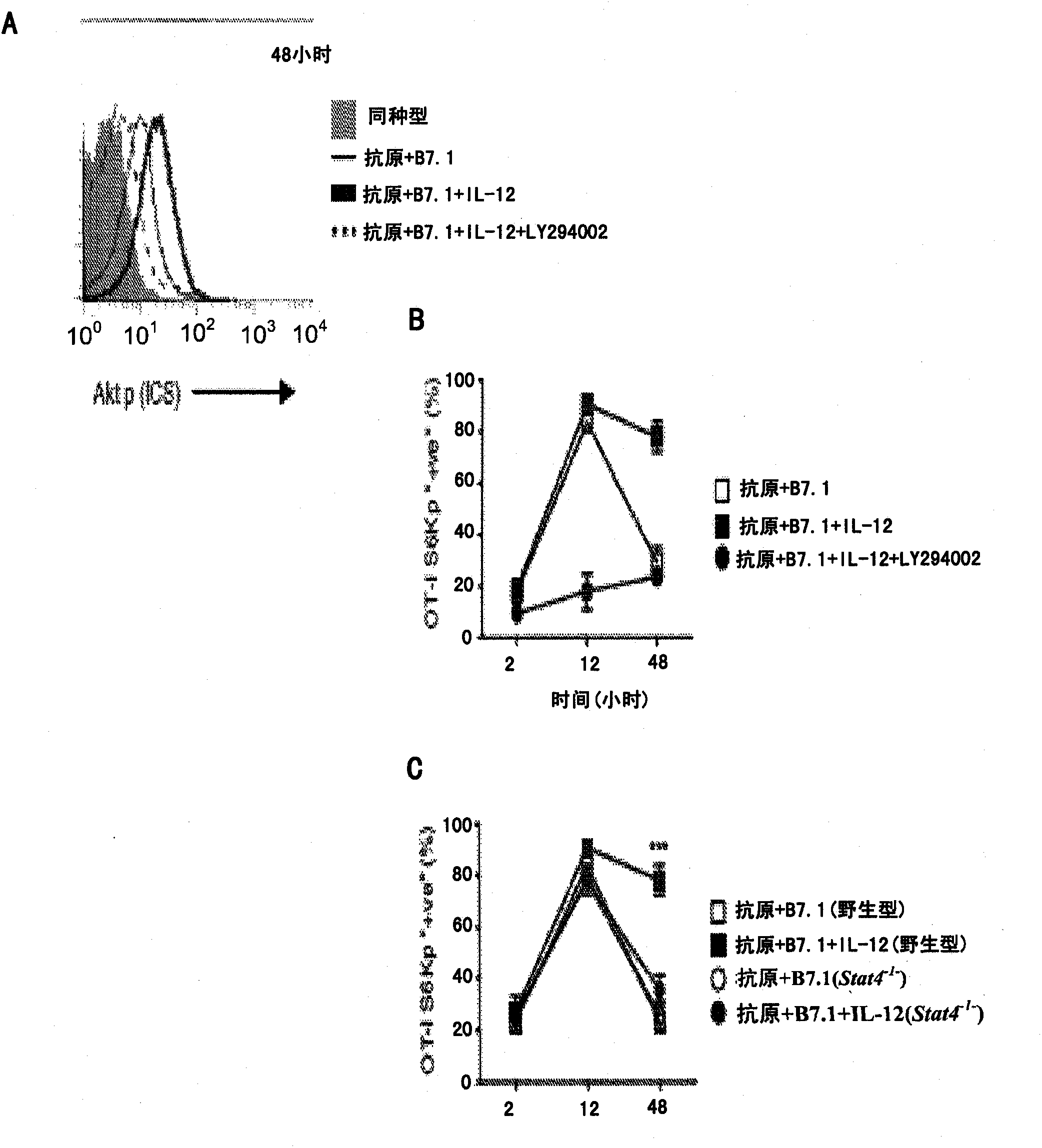
[0064] This example shows that in CD8 + IL-12 enhances mTOR activity in T cells requires PI3K and STAT4. To determine the control CD8 + Molecular pathways of mTOR activity in T cells, we analyzed the antigen-, B7.1- and IL-12-induced phosphoinositide 3-kinase (PI3K)-Akt kinase pathway for CD8 + Required for mTOR signaling in T cells. Akt phosphorylation (Thr308) in antigen + B7.1 ± IL-12 stimulated OT-I cells was assessed as a functional measure of PI3K activity. While stimulation with antigen+B7.1 for 30 min with or without IL-12 induced similar amounts of Akt phosphorylation, Akt phosphorylation was enhanced up to 48 h in the presence of IL-12, which was controlled by a PI3K inhibitor (LY294002 ) suppressed by ( figure 2 A), thus confirming that IL-12 enhanced antigen+B7.1-induced PI3K activity in OT-I cells. Furthermore, inhibition of PI3K blocked IL-12-enhanced mTOR activity (S6K phosphorylation was observed at 2, 12 and 48 hours) ( figure 2 B), showing that antige...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com