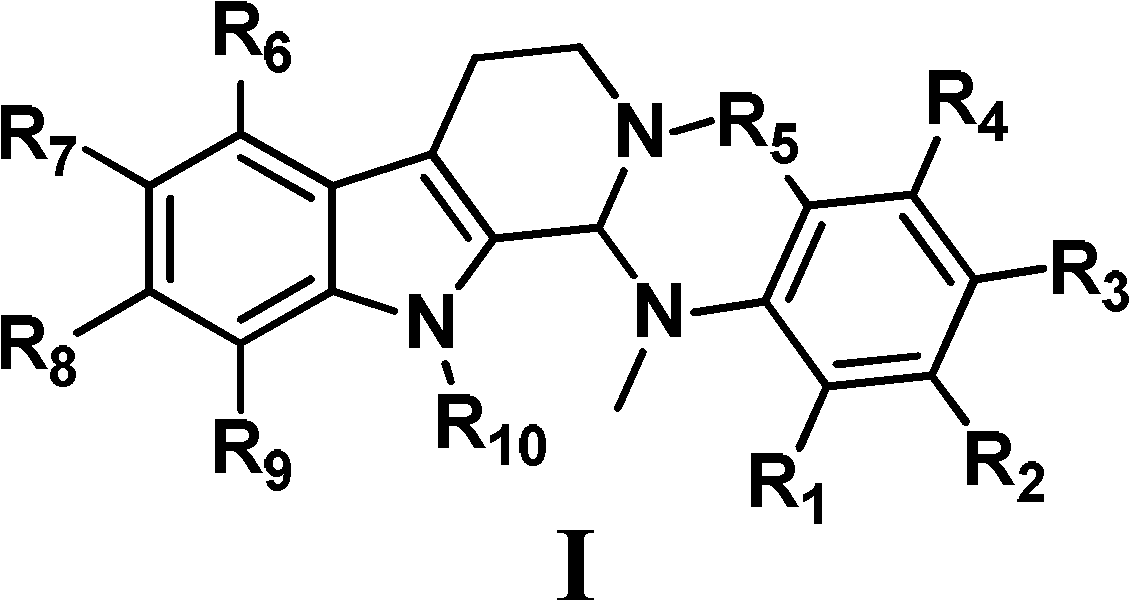
Evodiamine compounds, preparation method thereof and application thereof
A technology of evodiamine and chloroevodiamine, which is applied in the field of medicine, can solve the problems of high toxicity, poor water solubility and the like, and achieves the effect of good antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
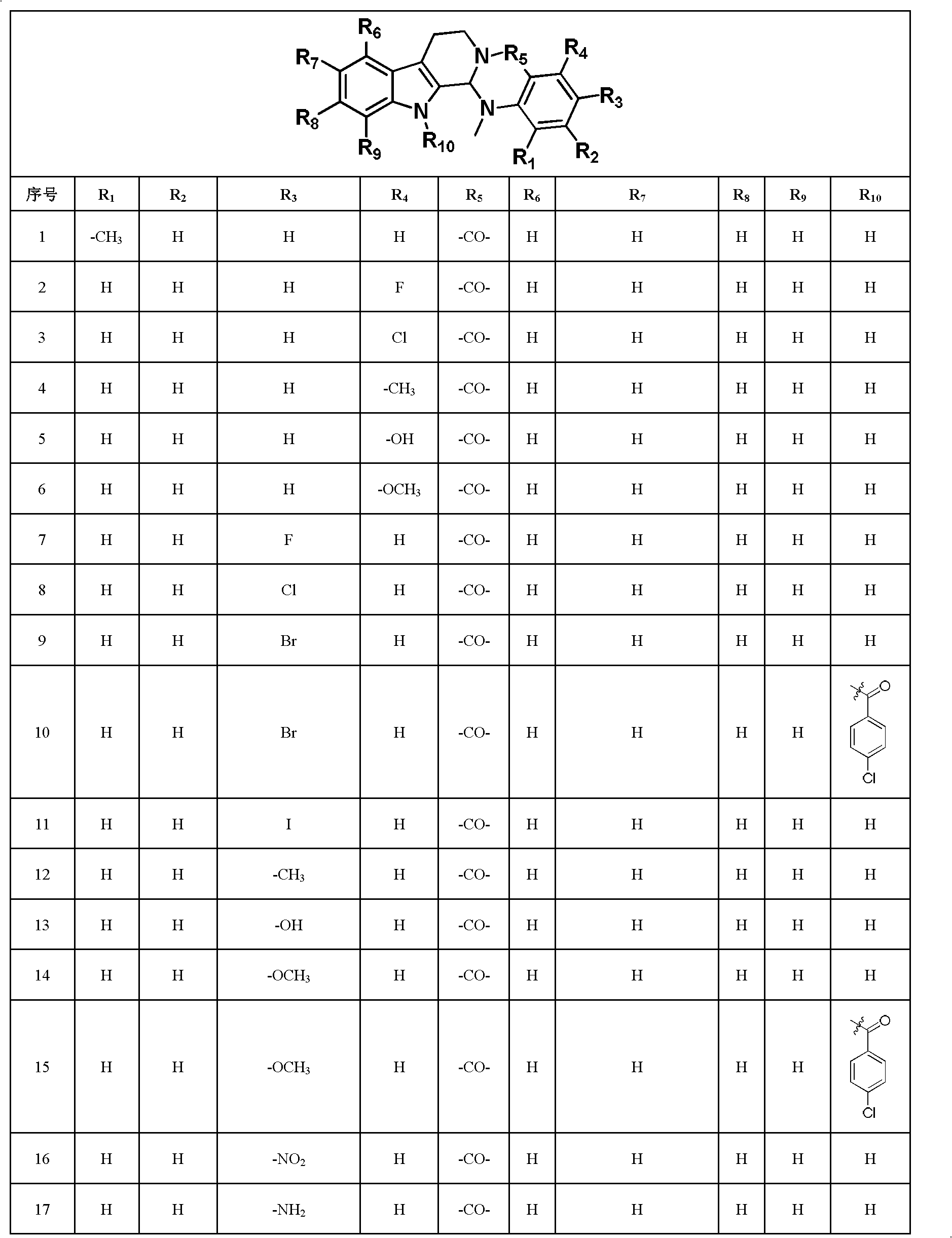
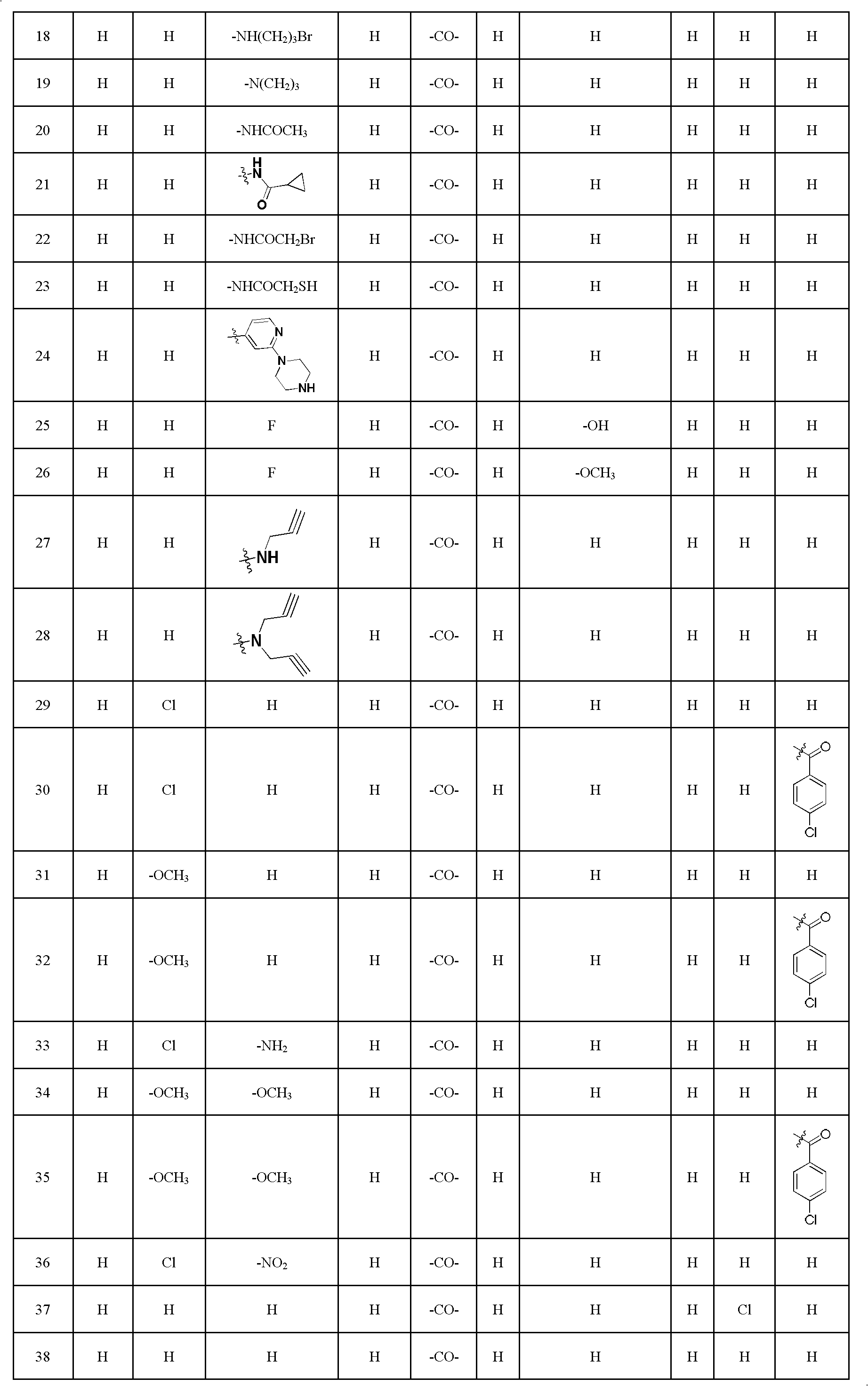
[0060] Embodiment 1: the synthesis of 1-methyl evodiamine
[0061] A. Preparation of N-formyltryptamine
[0062] In a 50ml three-necked bottle, add 8g (50mmol) of tryptamine and 25g of ethyl formate, and reflux at 80°C for 12 hours. After the reaction, the solvent is evaporated to dryness to obtain a brown oil, which is left at room temperature for 2-3 days, and crystals slowly appear. Suction filtration obtained 7.3 g of the product with a yield of 87.1%.
[0063] B. Preparation of 3,4-dihydro-β-carboline
[0064] In a 100ml three-neck flask, add 50ml of dichloromethane, under stirring conditions, add 5g (26mmol) N-formyl tryptamine, cool to about 5°C in an ice-water bath, then slowly add 12.5ml of phosphorus oxychloride, ice React in the bath for 2 hours, and then react at room temperature for 2 hours. After the reaction, dichloromethane and unreacted phosphorus oxychloride were recovered by distillation under reduced pressure, and the residual solid was extracted three ti...
Embodiment 2
[0071] Example 2: Synthesis of 4-fluoroevodiamine
[0072] According to the method of Example 1, in step C, 6-fluoroanthranilic acid is used instead of 3-methylanthranilic acid to obtain 5-fluoropyridine red acid anhydride, and in step D, 5-fluoropyridine red acid anhydride is used instead of 8- Methylidine red anhydride to get N-methyl-5-fluoropyridine red anhydride, replace N-methyl-8-methylpyridine red anhydride with N-methyl-5-fluoropyridine red anhydride in step E to get gray powder 0.47g of 4-fluoroevodiamine, the total yield is 36.5%.
Embodiment 3
[0073] Embodiment 3: the synthesis of 4-chloroevodiamine
[0074] According to the method of Example 1, in step C, 3-methylanthranilic acid is replaced with 6-chloroanthranilic acid to obtain 5-chloropyridine red acid anhydride, and in step D, 5-chlororidine red acid anhydride is used to replace 8- Methylidine red anhydride to get N-methyl-5-chloropyridine red anhydride, in step E replace N-methyl-8-methylpyridine red anhydride with N-methyl-5-chlororidine red anhydride to get gray powder 0.64g of 4-chloroevodiamine, with a total yield of 47.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com