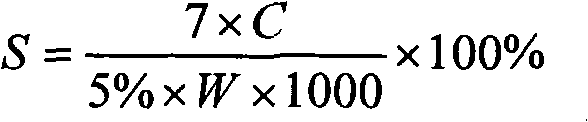Lidocaine hydrochloride transdermal ointment and preparation method thereof
A lidocaine hydrochloride transdermal technology, which is applied in the field of medicine, can solve the problems of low skin penetration rate of lidocaine, and achieve the effects of low price, high antioxidant capacity, and good skin softening properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
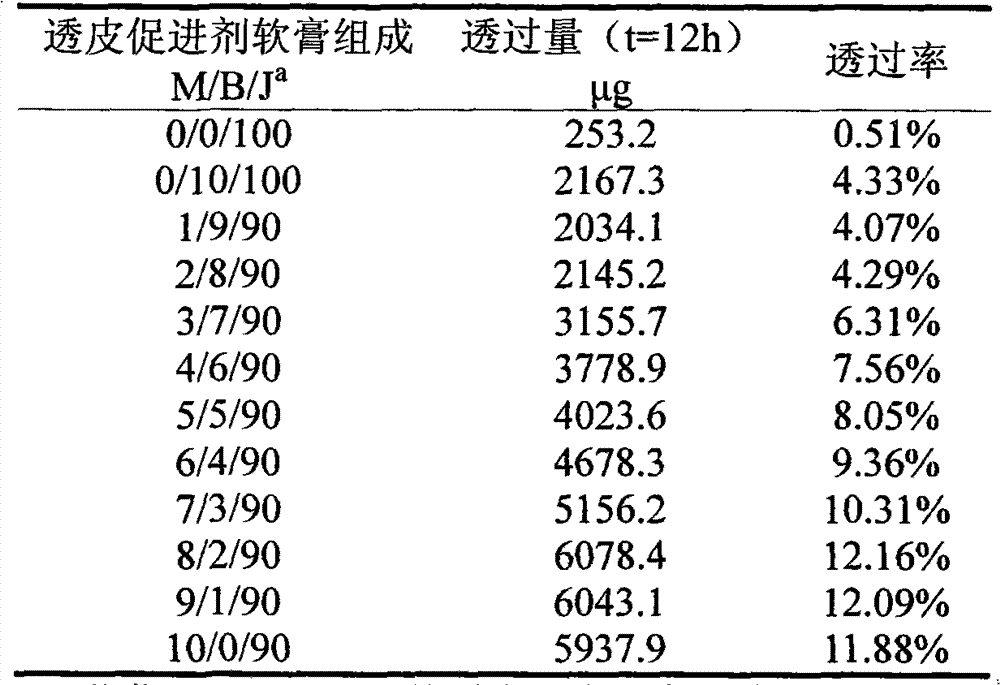
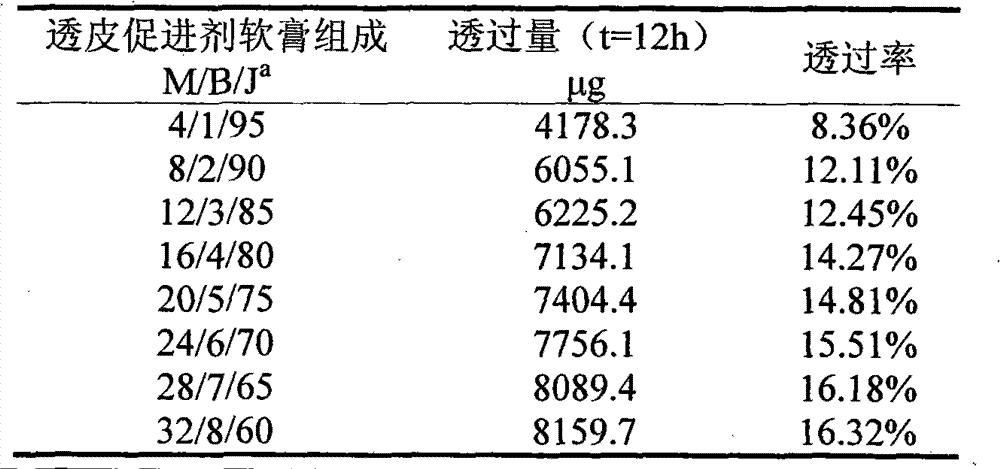
[0037] A lidocaine hydrochloride transdermal ointment is composed of a lidocaine hydrochloride ointment and a transdermal accelerator, wherein the transdermal accelerator includes mango butter and menthol.
[0038] The preparation method of lidocaine hydrochloride transdermal ointment, its steps are as follows:
[0039] (1) Mix 8g of mango butter, 2g of menthol, 1.4g of glyceryl monostearate, 2.4g of liquid paraffin, 0.4g of vaseline, and 2g of lanolin, and heat to 80°C to melt after mixing to make oil Mutually;
[0040] (2) Under the condition of 80° C., 0.16 g of triethanolamine, 3 g of lidocaine hydrochloride, and 0.04 g of ethylparaben were dissolved in 40 g of water to make an aqueous phase;
[0041] (3) Pour the water phase of step (2) into the oil phase of step (1), and make lidocaine hydrochloride transdermal ointment after stirring.
Embodiment 2
[0043] A lidocaine hydrochloride transdermal ointment is composed of a lidocaine hydrochloride ointment and a transdermal accelerator, wherein the transdermal accelerator includes mango butter and menthol.
[0044] The preparation method of lidocaine hydrochloride transdermal ointment, its steps are as follows:
[0045] (1) Heat 8g of mango butter, 2g of menthol, 2g of glyceryl monostearate, 4g of liquid paraffin, 1g of petrolatum, and 4g of lanolin to 70-80°C and mix and melt to make an oil phase ;
[0046] (2) Under the condition of 80° C., 0.2 g of triethanolamine, 5 g of lidocaine hydrochloride, and 0.1 g of ethylparaben were dissolved in 75 g of water to prepare an aqueous phase;
[0047] (3) Pour the water phase of step (2) into the oil phase of step (1), and make lidocaine hydrochloride transdermal ointment after stirring.
Embodiment 3
[0049] A lidocaine hydrochloride transdermal ointment is composed of a lidocaine hydrochloride ointment and a transdermal accelerator, wherein the transdermal accelerator includes mango butter and menthol.
[0050] The preparation method of lidocaine hydrochloride transdermal ointment, its steps are as follows:
[0051] (1) 6g of mango butter, 1g of menthol, 1g of glyceryl monostearate, 2g of liquid paraffin, 0.2g of vaseline, and 1g of lanolin are heated to 70°C and mixed and melted to form an oil phase;
[0052] (2) Under the condition of 70° C., 0.1 g of triethanolamine, 2 g of lidocaine hydrochloride, and 0.02 g of ethylparaben were dissolved in 15 g of water to prepare an aqueous phase;
[0053] (3) Pour the water phase of step (2) into the oil phase of step (1), and make lidocaine hydrochloride transdermal ointment after stirring.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com