Use of cathepsin L inhibitor in preparation of radiotherapeutic sensitization drug
A cathepsin and inhibitor technology, applied in the field of radiotherapy sensitizing drugs, can solve the problem of radiosensitization effect of cathepsin L inhibitor, etc., and achieve the effect of overcoming the radioresistance of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
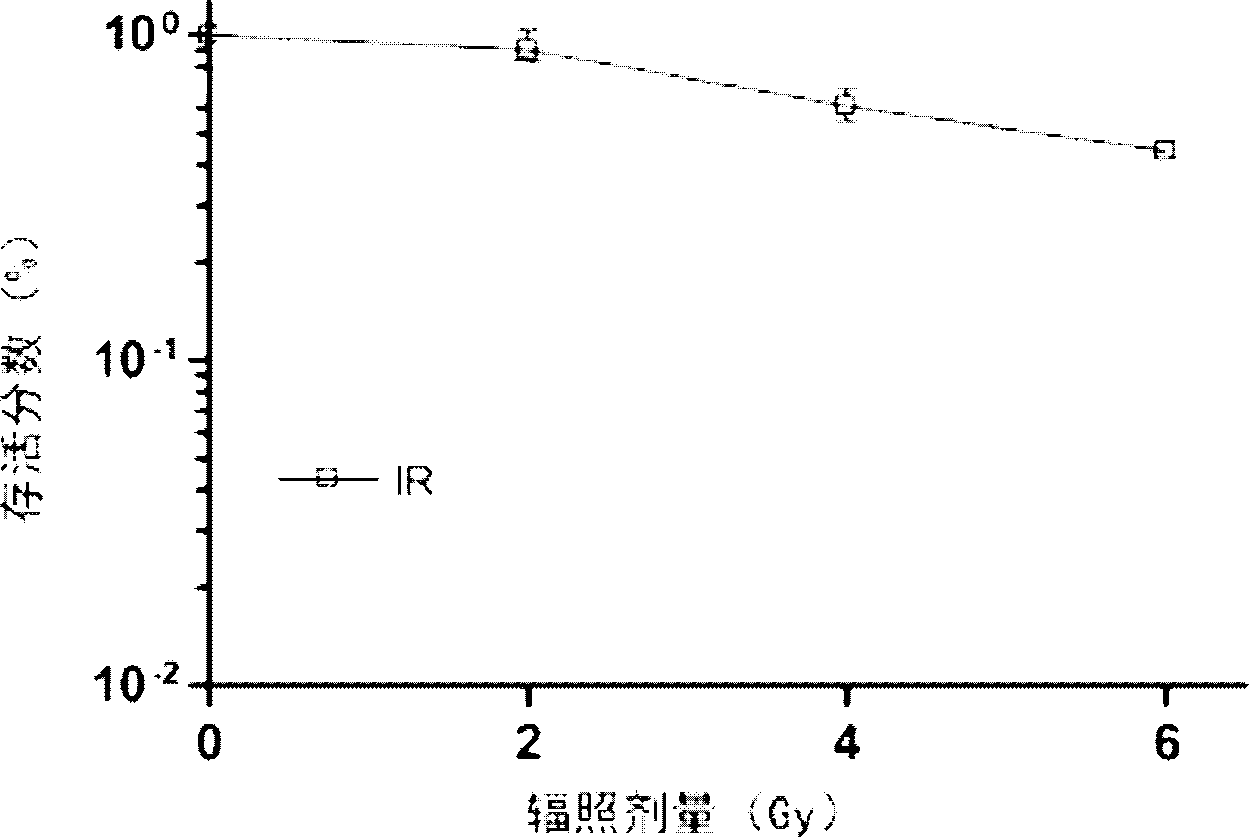
[0043] (1) Establish an in vitro culture system of human glioma stem cells and an orthotopic xenograft model in nude mice. The orthotopic tumor transplantation model in nude mice is a routine technique in the laboratory. Glioma stem cells in the logarithmic growth phase are taken for use. Take a 5-week-old nude mouse for anesthesia, cut the skin of the head, and use a bone drill to place it on the skull of the nude mouse Drill the hole, fix the nude mouse with a mouse stereotaxic instrument, and slowly inject the cells into the nude mouse brain with a micro-injection pump, the cell volume is 1×10 6 , slowly pull out the needle, and suture the wound.
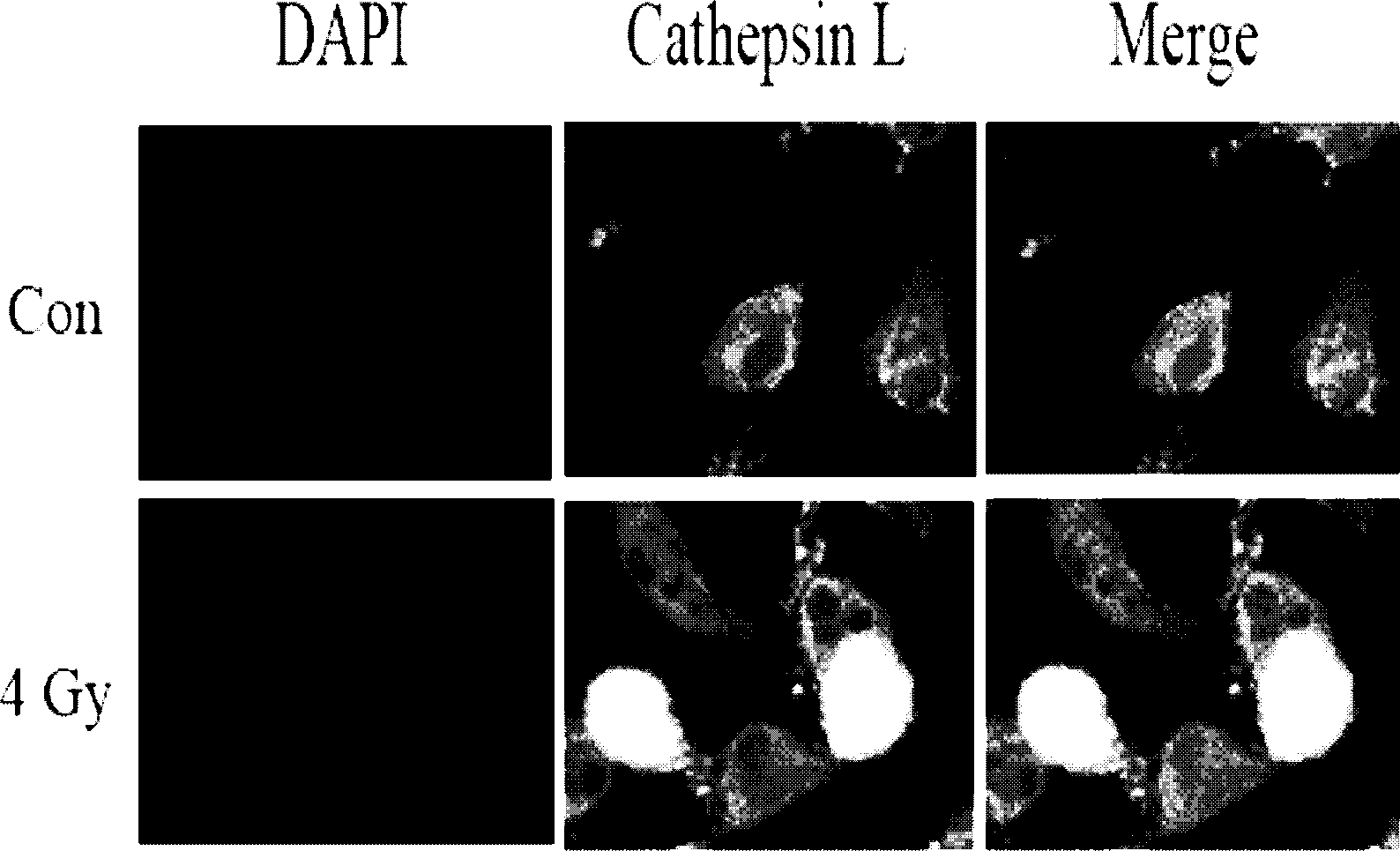
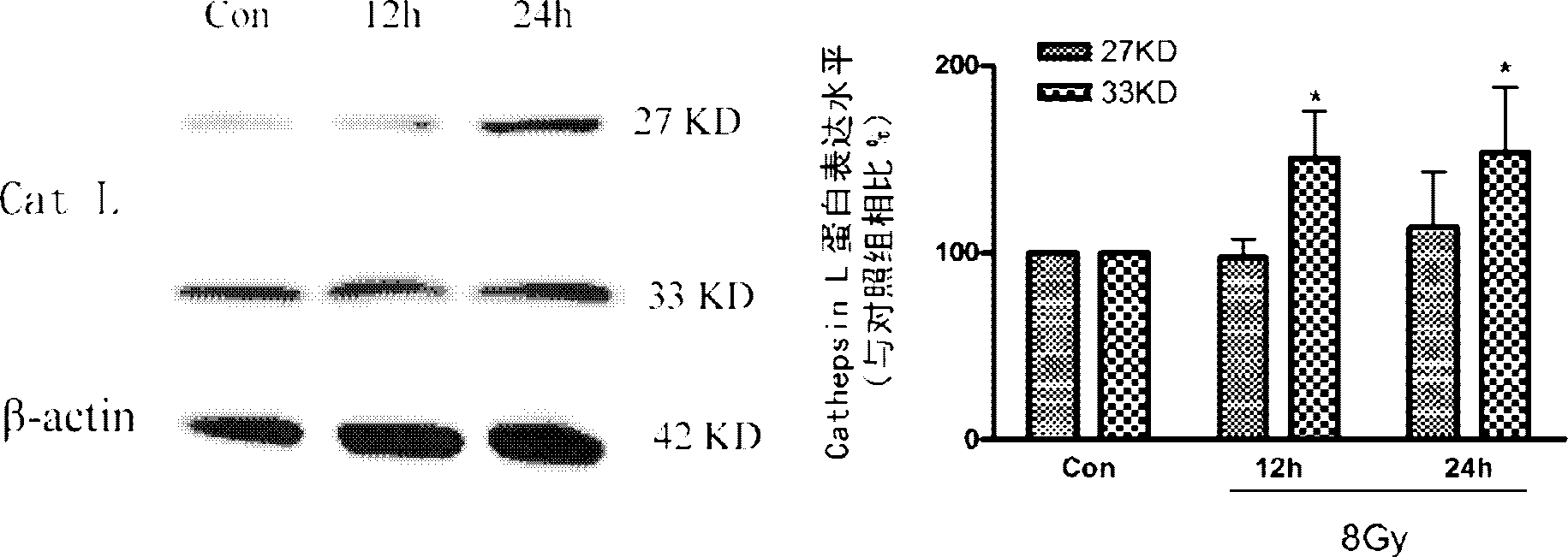
[0044] (2) Immunofluorescence and Western blot were used to observe the changes in the expression of Cathepsin L in glioma stem cells after X-ray treatment:
[0045] Immunofluorescence method treatment steps: take logarithmic growth phase glioma stem cells, inoculate them in 6-well cell culture plates, and give 6Gy X-ray treatme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com