Quality control method of traditional Chinese medicine preparation for treating rheumatism
A technology for traditional Chinese medicine preparations and rheumatism, which is applied in the field of quality control of traditional Chinese medicine preparations, and can solve problems such as incurable, life-threatening, disability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0063] Specific embodiments of the identification of Acanthopanax
[0064] Take 0.7g of the content of this product, grind it finely, add 20ml of methanol, ultrasonically treat it for 20 minutes, filter, put the filtrate on a water bath and evaporate to dryness, add 1ml of methanol to the residue, and use it as the test solution, and take 5g of Acanthopanax as a reference medicinal material, Add 50ml of 75% ethanol, heat to reflux for 1 hour, let cool, filter, evaporate the filtrate to dryness on a water bath, add 10ml of water to the residue to dissolve, add chloroform to extract twice, 10ml each time, combine the chloroform solution, evaporate to dryness on a water bath, Add 1ml of methanol to the residue to dissolve, as the reference medicinal material solution, then take the reference substance of isoferidin, add methanol to make a solution containing 1mg per 1ml, as the reference solution, test according to thin-layer chromatography, draw 10ul of each of the above three so...
specific Embodiment 2
[0065] Specific embodiment two microscopic identification
[0066] Take the content of the present invention and put it under a microscope to observe: the stone cells are colorless, brown in appearance connected with the epigenetic cortex, sub-square, sub-rectangular, sub-circular, sub-prismatic or strip-shaped, 60um in diameter, 100um in length, slightly walled. Thick, the thicker wall has obvious layers, the fibers are mostly in bundles, light yellow, in the shape of slender strips, the end is short and pointed, and some short branches can be seen, the edge is not smooth, the diameter is 40um, the wall thickness is 6um, and there are red bordered cells Brown, polygonal, thin-walled, phloem parenchyma cells spindle-shaped, slightly thicker wall, with very fine oblique staggered texture, epidermal cell fragments light yellow, rectangular cells, containing tiny calcium oxalate crystals.
specific Embodiment 3
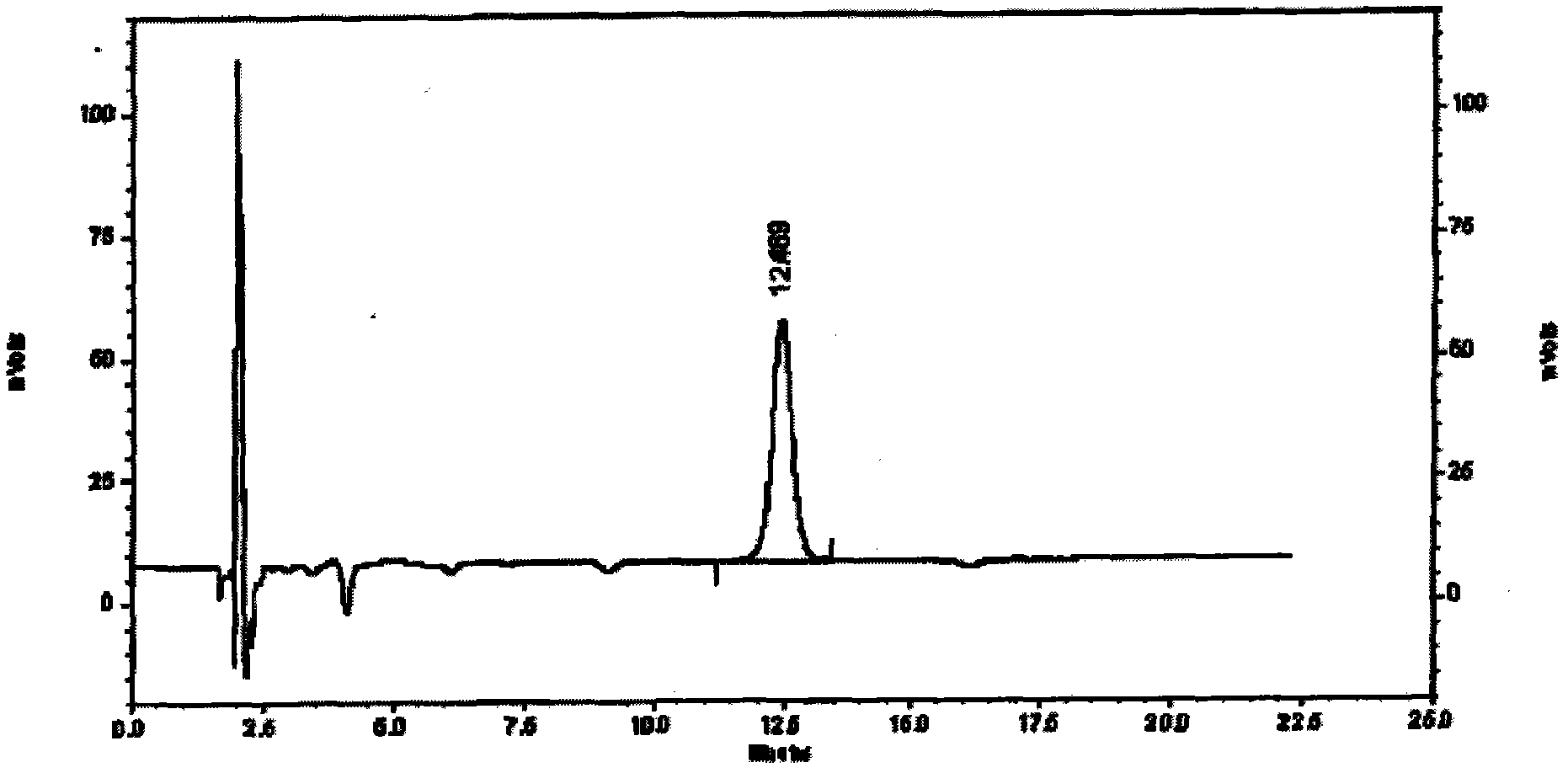
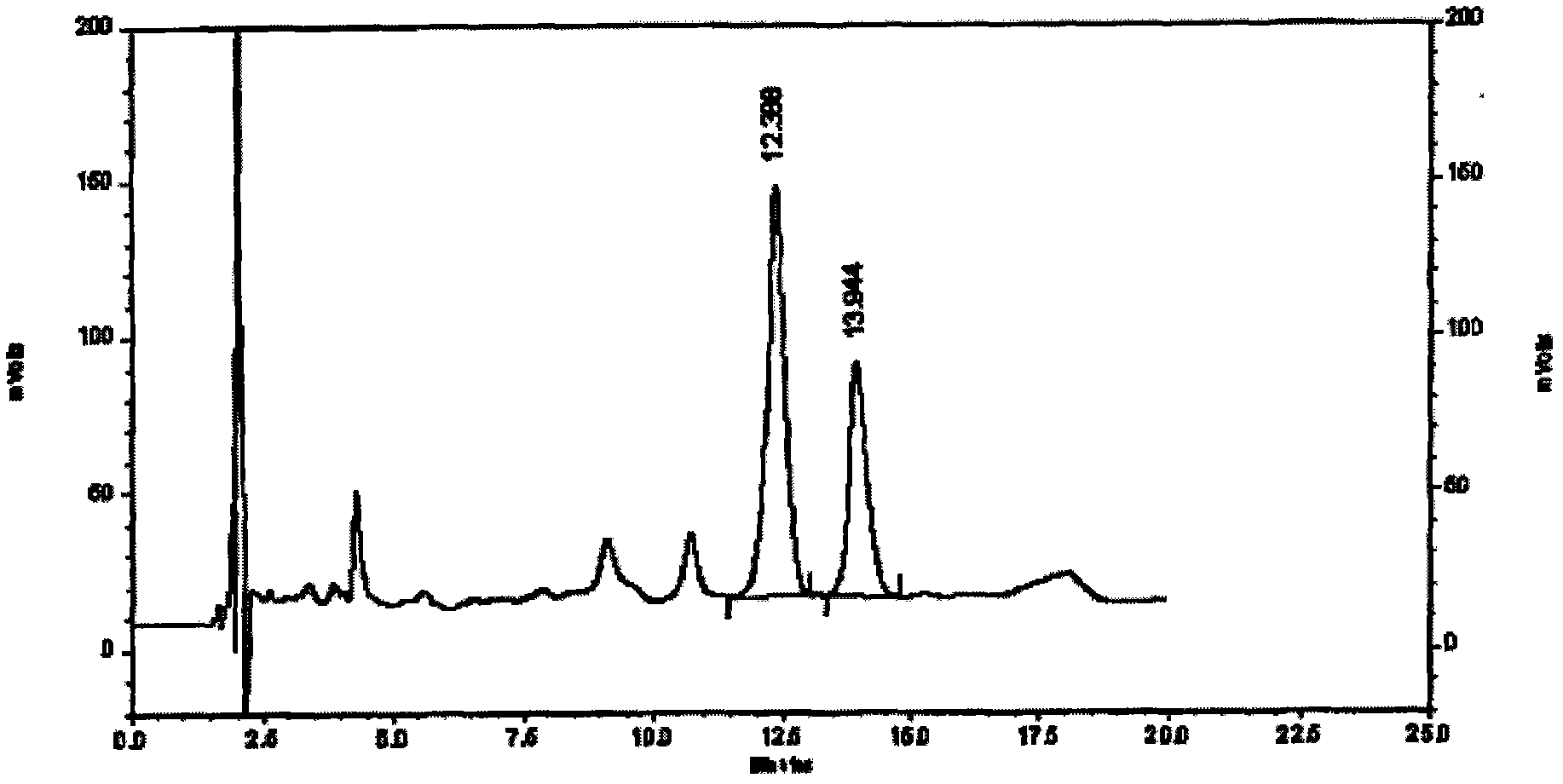
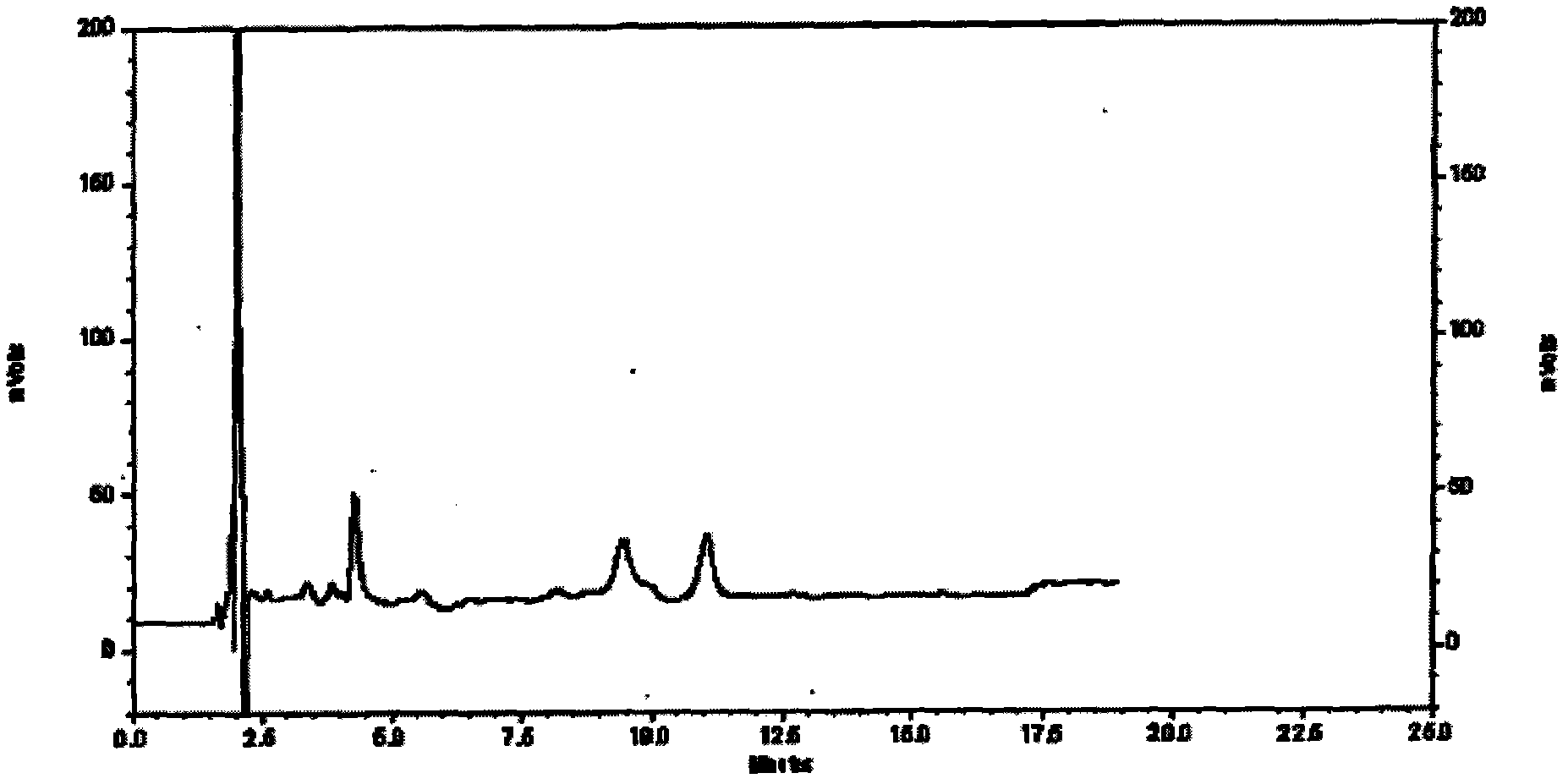
[0067] Specific embodiment three assays
[0068] Content determination is determined according to high performance liquid chromatography;
[0069] Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler, and acetonitrile-0.1% phosphoric acid solution with a ratio of 7:93 as mobile phase: the detection wavelength is 207nm, and the number of theoretical plates is based on ephedrine hydrochloride Peak calculation should not be less than 3000,
[0070] Preparation of reference substance solution Precisely weigh an appropriate amount of ephedrine hydrochloride reference substance, add methanol to make a solution containing 20ug in every 1ml, to obtain final product,
[0071] The preparation of need testing solution takes 3.5g of the content of the present invention, is precise and stable, grinds finely, gets about 1g, accurately weighs, puts conical flask, adds 60% ethanol 50ml, ultrasonic 30 minutes, let cool, filter, use Wash the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap