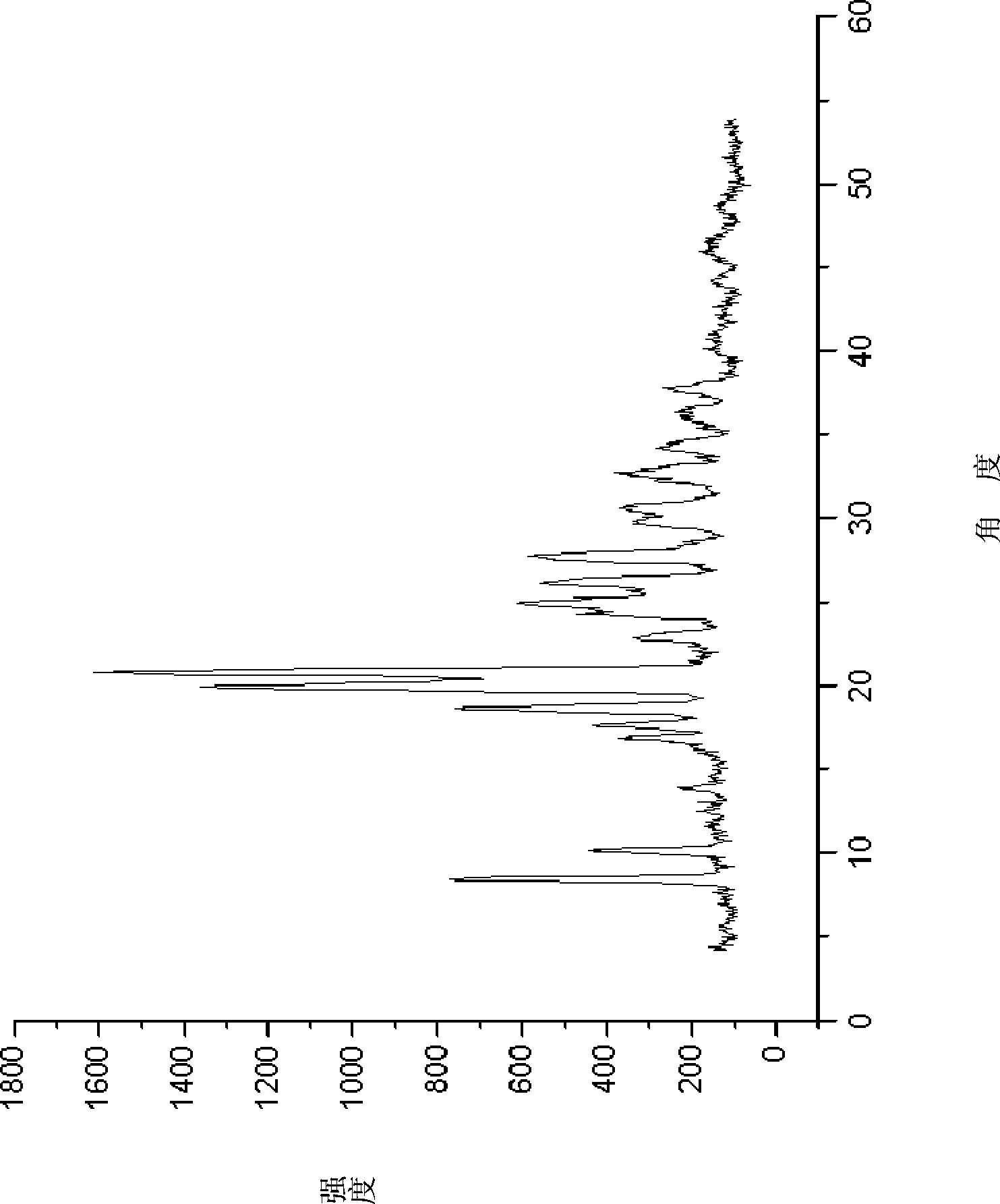
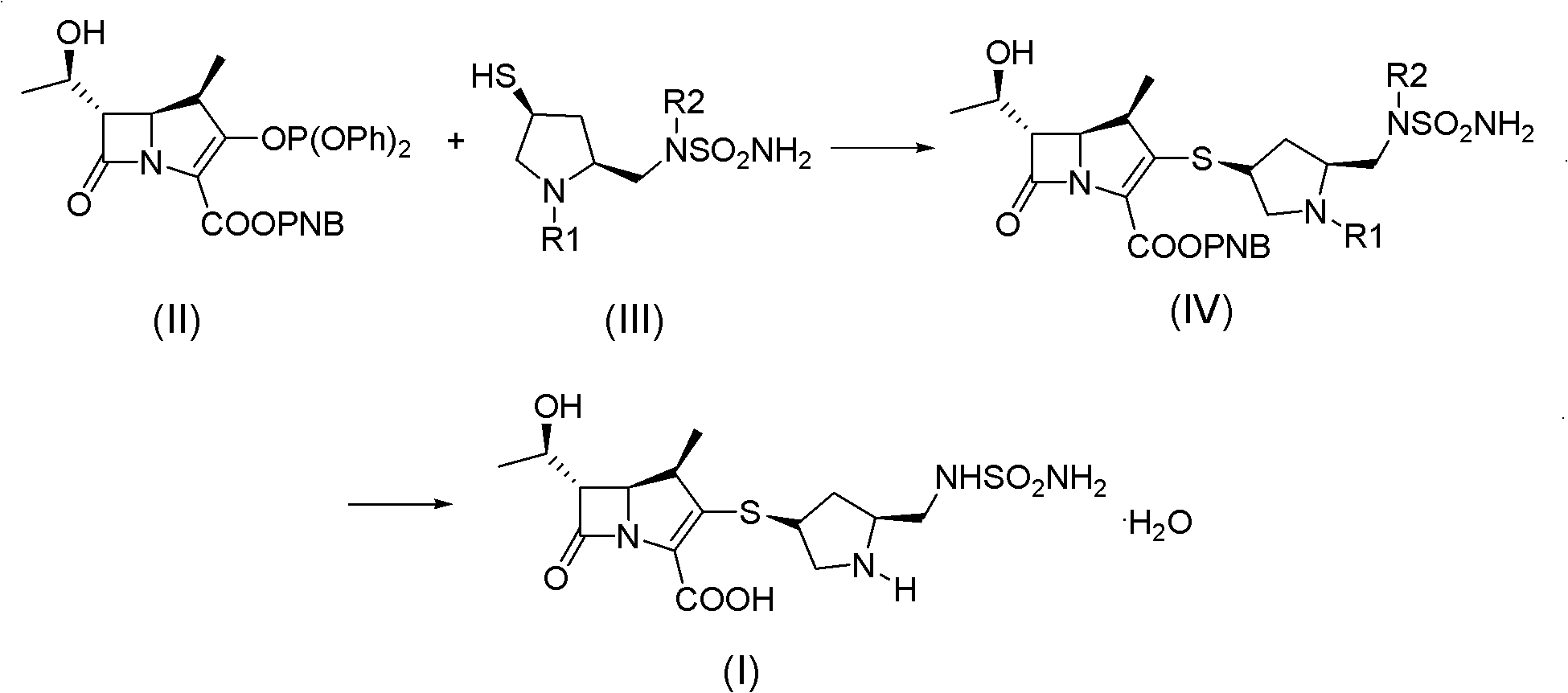
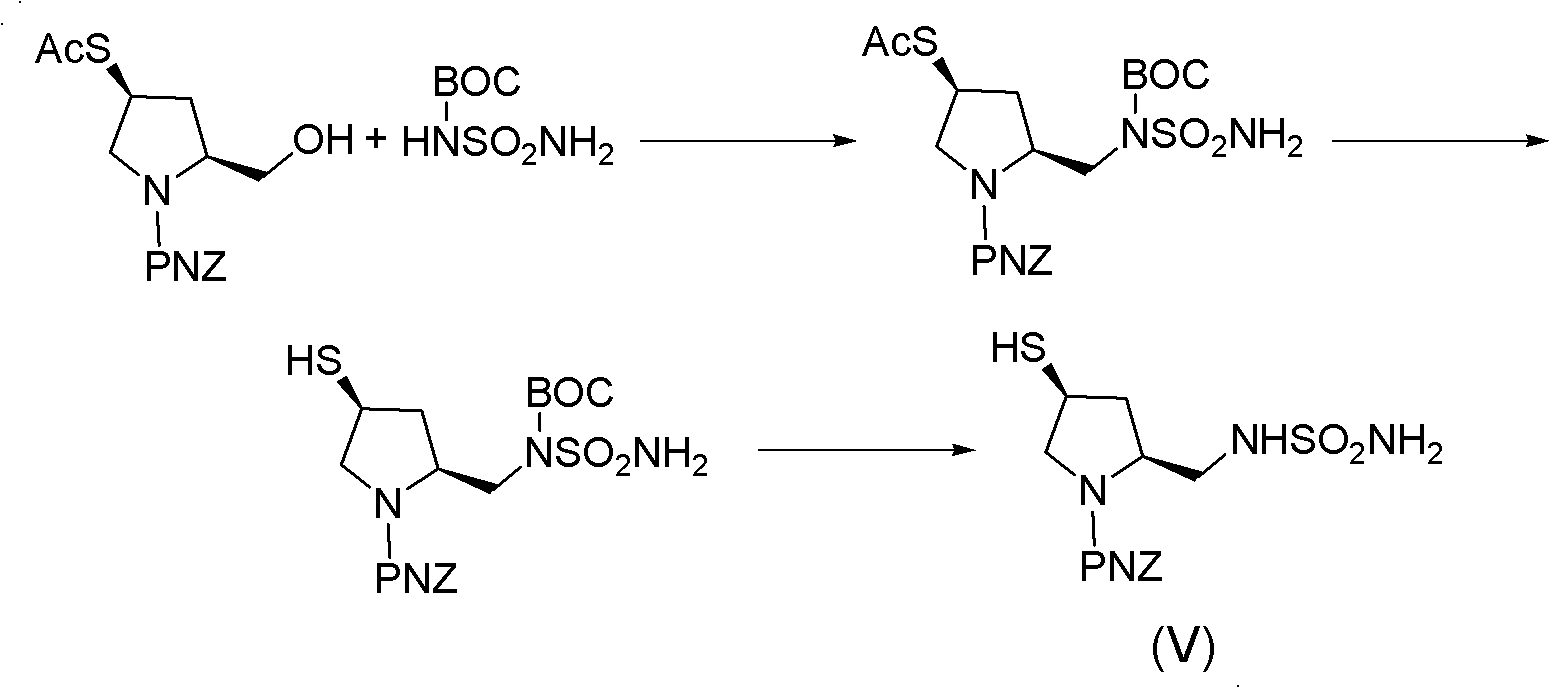
Doripenem side-chain compound and preparation method and application thereof
A chain compound and compound technology, applied in the field of doripenem side chain compound and its preparation, can solve the problems of increasing side chain manufacturing costs, troubles, hidden dangers of final product purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] The synthesis of embodiment 1 compound (XII)
[0089] At room temperature, 16.54g (0.06mol) of the compound of formula (X) was dissolved in 125ml of tetrahydrofuran, and 20.8g (0.08mol) of triphenylphosphine and 0.08mol of the compound of formula (XI) were added. Add 16 g (0.08 mol) of diisopropyl azodicarboxylate (DIAD), and control the rate of addition so that the reaction temperature is not higher than 0°C. After the addition is complete, warm up to room temperature and continue stirring. HPLC monitors that the reaction is complete, and the solvent is evaporated under reduced pressure, 100ml of toluene is added, cooled to 0°C, stirred for 4 hours, filtered, and the filter cake is washed several times with 50ml of toluene cooled to 0°C, and the filtrate is evaporated under reduced pressure to obtain the formula ( XIII) Crude compound. The obtained crude product was directly put into the next step reaction without further purification.
[0090] (2S,4S)-1-tert-butox...
Embodiment 2
[0093] The synthesis of embodiment 2 compound (XII)
[0094] At room temperature, 16.54g (0.06mol) of the compound of formula (X) was dissolved in 125ml of toluene, and 20.8g (0.08mol) of triphenylphosphine and 0.08mol of the compound of formula (XI) were added. Add 16 g (0.08 mol) of diisopropyl azodicarboxylate (DIAD), and control the rate of addition so that the reaction temperature is not higher than 0°C. After the addition is complete, warm up to room temperature and continue stirring. The completion of the reaction was monitored by HPLC, the temperature was lowered to 0° C., stirred for 4 hours, filtered, and the filter cake was washed several times with 50 ml of toluene cooled to 0° C., and the filtrate was evaporated under reduced pressure to remove the solvent to obtain a crude compound of formula (XII). The obtained crude product was directly put into the next reaction without further purification.
[0095] (2S,4S)-1-tert-butoxycarbonyl-4-acetylthio-2-(N-tert-buty...
Embodiment 3
[0098] The synthesis of embodiment 3 compound (XIII)
[0099] Under nitrogen protection, stir and dissolve 3g (0.072mol) of sodium hydroxide in 80ml of water and 12ml of methanol mixed solvent, cool to 0°C, dropwise add the crude product of formula (XII) compound (containing 0.024mol of formula (XII) compound) After adding 12ml of dichloromethane solution and a few drops of tributylphosphine, continue to stir for 2 hours, HPLC monitors the completion of the reaction, add dichloromethane to the reaction solution under nitrogen protection, extract 2 times, 40ml each time, collect the water phase and cool To -5 ~ 0 ° C, under the protection of nitrogen, add 2M hydrochloric acid to the pH of the solution to 4 ~ 6, control the drop rate of hydrochloric acid, so that the solution temperature does not exceed 0 ° C, add dichloromethane to extract 3 times, 50ml each time, combine the organic phase , added anhydrous sodium sulfate to dry for 2 hours, filtered, and distilled off the so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com