Modulation of inflammatory responses by factor xi
A technology for factors and inflammatory diseases, applied in the field of regulation of inflammatory responses by factor XI, can solve problems such as harmful side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
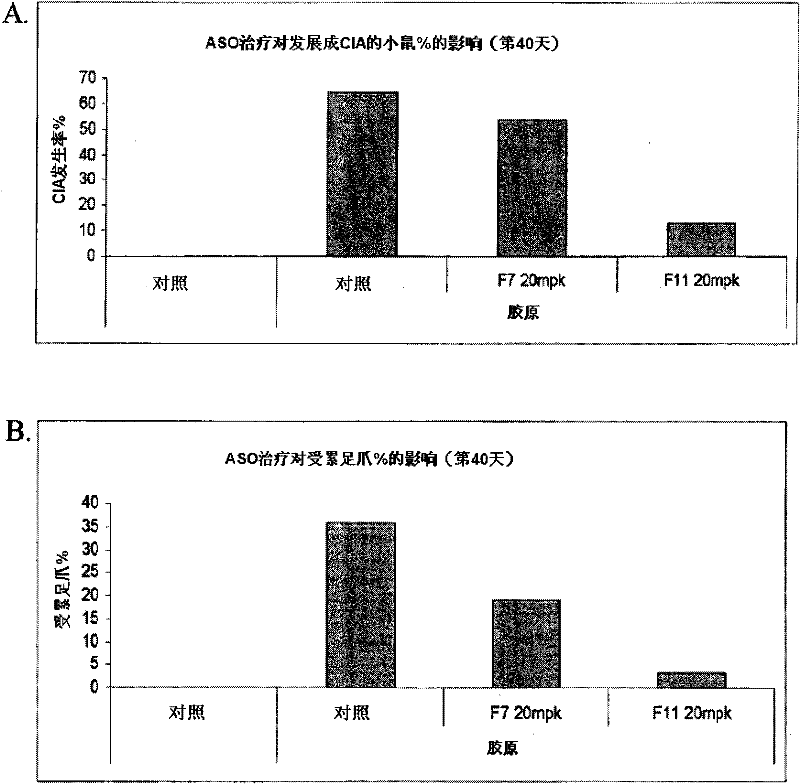
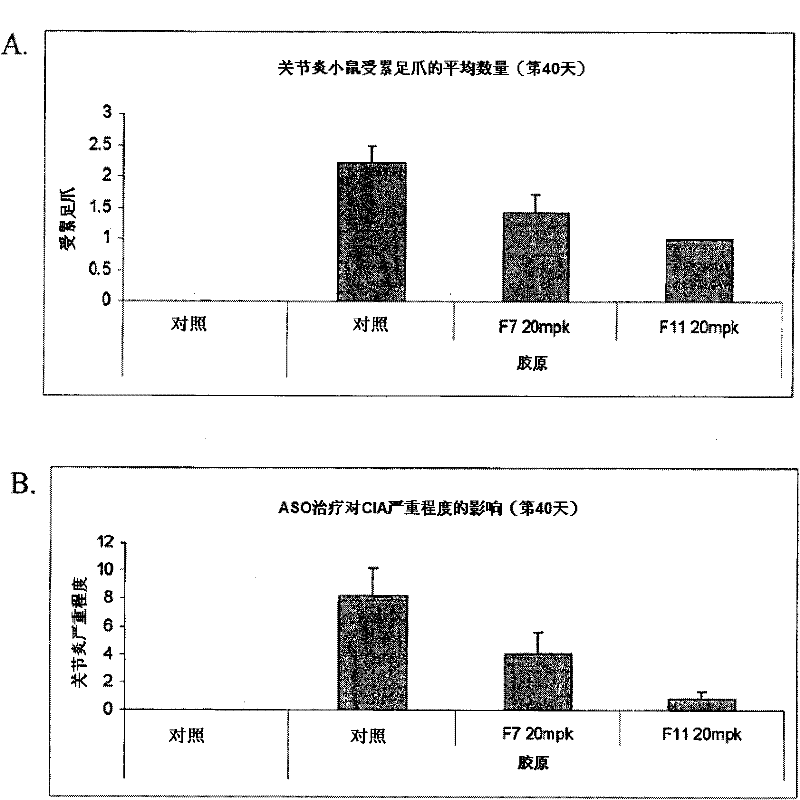
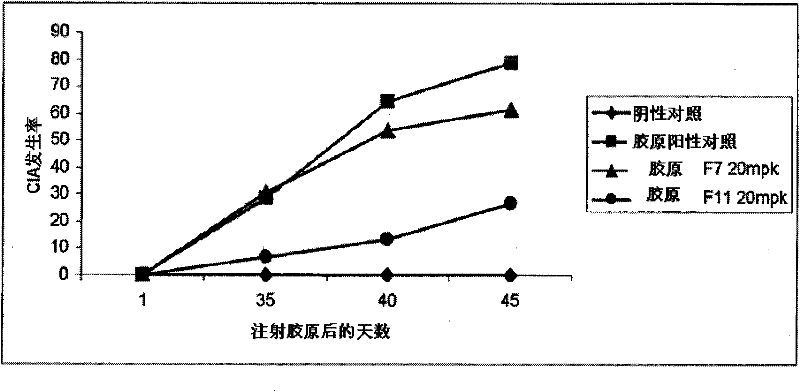
[0123] In certain embodiments, methods, compounds and compositions are provided for administering a compound to an animal to modulate an inflammatory response, wherein the compound comprises a modulator of Factor XI. Modulation of Factor XI can result in an increase or decrease in Factor XI mRNA and protein expression to increase or decrease a desired inflammatory response. In certain embodiments, inhibition of Factor XI in an animal can be reversed by administering a modulator that targets Factor XI. In certain embodiments of the invention, Factor XI is inhibited by a modulator. Factor XI modulators may be modified oligonucleotides targeted to Factor XI.
[0124] In certain embodiments, provided are methods, compounds and compositions for treating, preventing or ameliorating Factor XI-associated inflammatory diseases, disorders and conditions in an animal in need thereof. In one embodiment, a method of ameliorating an inflammatory disease in an animal comprises administerin...
Embodiment 1
[0425] Example 1: Antisense Inhibition of Human Factor XI in HepG2 Cells
[0426]The effect of antisense oligonucleotides targeting Factor XI nucleic acids on Factor XI mRNA in vitro was tested. Cultured HepG2 cells at a density of 10,000 cells per well were transfected with liposome reagents containing 75 nM antisense oligonucleotides. After approximately 24 hours of treatment, RNA was isolated from the cells and levels of Factor XI mRNA were determined by quantitative real-time PCR. Factor XI mRNA levels according to RIBOGREEN The measured total RNA content was adjusted. Results are expressed as percent inhibition of Factor XI compared to untreated control cells.
[0427] The chimeric antisense oligonucleotides in Tables 1 and 2 were designed as 5-10-5 MOE gapmers. The gapmer is 20 nucleotides long, and the middle spacer contains 10 2'-deoxynucleotides and is connected to flanking regions containing 5 nucleotides on both sides (in the 5' and 3' directions). Every nucle...
Embodiment 2
[0438] Example 2: Dose-dependent antisense inhibition of human factor XI in HepG2 cells
[0439] Twelve gapmers were tested at different doses in HepG2 cells, and the inhibitory effects of these gapmers on human factor XI in vitro were all 84% or above. Cells were plated at a concentration of 10,000 cells per well and transfected with liposome reagents containing the antisense oligonucleotides shown in Table 3 at concentrations of 9.375 nM, 18.75 nM, 37.5 nM, 75 nM and 150 nM. After approximately 16 hours of treatment, RNA was isolated from the cells and the level of Factor XI mRNA was determined by quantitative real-time PCR. Human factor XI primer probe set RTS 2966 (forward sequence: CAGCCTGGAGCATCGTAACA, incorporated into the present invention as SEQ ID NO: 3; reverse sequence: TTTATCGAGCTTCGTTATTCTGGTT, incorporated into the present invention as SEQ ID NO: 4; probe sequence: TTGTCTACTGAAGCACACCCAAACAGGGAX, wherein X is a fluorophore, incorporated into the present inventi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com