PCR-ELISA based method for detecting mycobacterium tuberculosis resistance gene
A technology of Mycobacterium tuberculosis and drug-resistant genes, which is applied to the determination/testing of microorganisms, biochemical equipment and methods, etc., to achieve high sensitivity, increase sensitivity, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] 1. Preparation of template DNA:
[0029] For the DNA extraction of Mycobacterium tuberculosis in the specimen to be tested, take the clinical mycobacterium strain and place it in a 1.5 ml centrifuge tube, dissolve it in 0.5 ml TE solution, centrifuge at 14000 r / min for 5 min, discard the supernatant; add 100 μL of 1% TritonX-100 lysate, vortex to mix, 100 ° C water bath for 15 min, immediately ice bath for 3 min; 140000 r / min, 10 min, take the supernatant.
[0030] 2. PCR reaction:
[0031] a. Prepare 3×50 μL reaction system: In each reaction tube, 2 μL of primers rpoB-F and rpoB-R, 5 μL of 10×Buffer, 0.5 μL of template DNA, 2 μL of dNTPs, 2 U of Taq enzyme, the total volume is 50 μL. The target gene of the primer is rpoB gene (GenBank accession number: L27989)
[0032] The 5' ends of the downstream primers were all labeled with digoxigenin, and the sequences of the primers were:
[0033] rpoB-F 5'-GTGGTCGCCGCGATCAAG-3'
[0034] rpoB-R 5'-CACGTCGCGGACCTCCAG-3'
[0...
Embodiment 1
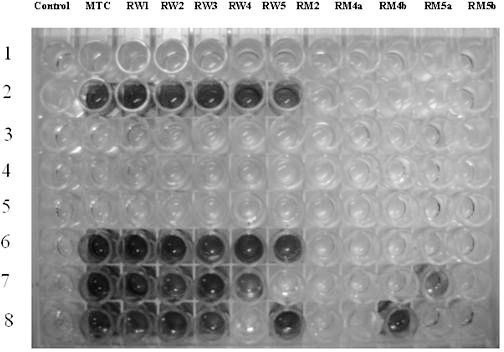
[0060] Specimens to be tested: 1 case of Escherichia coli strain, 1 case of Mycobacterium tuberculosis H37Rv strain, 3 cases of non-tuberculous mycobacteria were Mycobacterium avium, Mycobacterium intracellulare and Mycobacterium smegmatis; 1 case of clinical tuberculosis Mycobacterium rpoB gene wild-type strains, 2 cases of clinical Mycobacterium tuberculosis rpoB gene mutant strains.
[0061] 1. Preparation of template DNA:
[0062] Take the branched strain and put it in a 1.5 ml centrifuge tube, dissolve it in 0.5 ml TE solution, centrifuge at 14000 r / min for 5 min, discard the supernatant; add 100 μL of 1% TritonX-100 lysate, vortex to mix, and place in a 100°C water bath 15 min, immediately ice bath for 3 min; 140000 r / min, take the supernatant.
[0063] 2. PCR reaction:
[0064] a. Prepare 3×50 μL reaction system: In each reaction tube, 2 μL of primers rpoB-F and rpoB-R, 5 μL of 10×Buffer, 0.5 μL of template DNA, 2 μL of dNTPs, 2 U of Taq enzyme, the total volume is 50...
Embodiment 2
[0076] 1. Preparation of bacterial DNA 156 strains of Mycobacterium tuberculosis clinical isolates were derived from clinical inpatients. Mycobacterium tuberculosis was cultured in accordance with the "National Regulations for the Bacteriology of Diagnosis of Tuberculosis". Mycobacteria were initially identified and tested for drug susceptibility. Take clinical mycobacterium strains and place them in a 1.5 ml centrifuge tube, dissolve them in 0.5 ml TE solution, centrifuge at 14000 r / min for 5 min, discard the supernatant; add 100 μL of 1% TritonX-100 lysate, vortex to mix, and store at 100°C Water bath for 15 min, immediately ice bath for 3 min; 140000 r / min, take the supernatant.
[0077] 2. PCR amplification
[0078] a. Prepare 3×50 μL reaction system: In each reaction tube, 2 μL of primers rpoB-F and rpoB-R, 5 μL of 10×Buffer, 0.5 μL of template DNA, 2 μL of dNTPs, 2U of Taq enzyme, the total volume is 50 μL. The target gene of the primers is the mutation hotspot of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com