Composition for preventing or treating obesity-related diseases mediated by the activation of ampk and including 2,5-bis-aryl-3,4-dimethyltetrahydrofuran lignans as active ingredients
A technology of composition and lignans, applied in the direction of organic active ingredients, medical preparations containing active ingredients, active ingredients of heterocyclic compounds, etc., can solve the problem of increasing the number of fat cells and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
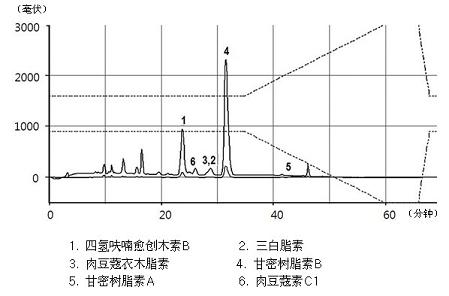
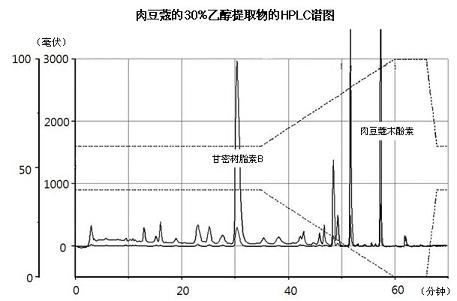
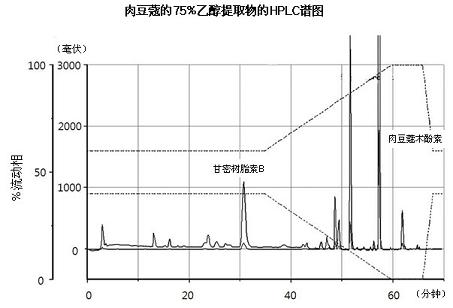
[0051] Powdered nutmeg (100 g) was dissolved in each solution (500 mL, see Table 1), and then the active substance was extracted 3 times within 2 hours using an ultrasonic extractor. Use the same concentration of the active ingredient of the isolated extract. Myristicin was obtained using Sigma (Cat. No. M9237). Each solution was used to extract the nutmeg extract and passed through MeOH / H 2 O (0-32 min: 63% MeOH, 32-37 min: 63 → 100% MeOH) for analysis of myristyl ether. Table 1 shows the contents of myristicin and carmasin B in nutmeg extracts extracted using different solutions.
[0052] Table 1
[0053]
[0054]As shown in Table 1, the 30% ethanol extract contained an average of 0.45% myristicin, about one-third of the 1.27% of the 75% ethanol extract, and 1.85% of the 75% methanol extract under the same conditions . Also, using MeOH / H 2 O (0-35 minutes: 60% MeOH, 35-60 minutes: 60→100% MeOH) HPLC (Optima Pak C18 column 4.6x250mm, particle size 5μm, flo...
Embodiment 2
[0056]
[0057] Nutmeg (500 g) was extracted using 30% aqueous ethanol (1,000 mL) and passed through ion exchange resin Diaion HP-20 to adsorb onto the resin (500 g). Then, the extract was eluted with 1 liter of 50% ethanol, 60% ethanol, 70% ethanol, 80% ethanol, 90% ethanol, 100% ethanol and 100% acetone, respectively. Table 2 shows the degree of elution of calmasin B and myristicin adsorbed on Diaion HP-20 by ethanol solution and acetone.
[0058] Table 2
[0059]
[0060] As shown in Table 2, myristicin was hardly detected when the substances adsorbed on Diaion HP-20 were eluted with 80% or less ethanol.
[0061] The same solvent was used to monitor the elution of glabrinin B, one of the 2,5-diaryl-3,4-dimethyltetrahydrofuranolignans. As in Table 2, Figure 5 and Figure 6 As shown, when 80% ethanol was used as the eluent, the concentration of eluted calmaxinin B was the highest.
Embodiment 3
[0062]
[0063] In Example 1, using 1 H-and 13 C-NMR analysis of 2,5-diaryl-3,4-dimethyltetrahydrofuranolignans isolated from 30% ethanolic Myristica extract by HPLC. The physical and chemical properties of 2,5-diaryl-3,4-dimethyltetrahydrofuranolignans (compounds 1-6) extracted from nutmeg of the present invention are as follows.
[0064] 3-1. Tetrahydrofuran guaiacin (compound 1)
[0065] colorless powder; 1 H-NMR: ppm (500 MHz, CDCl 3 ): δ0.61 (6H, d, J = 6.0 Hz, 3- and 4-Me), 2.67 (2H, m, 3- and 4-H), 3.91 (6H, 3'- and 3"-OMe) , 5.12 (2H, d, J = 6.6 Hz, 2- and 5-H), 5.59 (2H, s, 4'- and 4"-OH), 6.90-6.99 (6H, m, 2'-, 5' -, 6'-, 2"-, 5"- and 6"-H); 13 C-NMR: ppm (125 MHz, CDCl 3 ): δ11.7 (3- and 4-Me), 41.5 (C-3 and C-4), 55.8 (2×OMe), 82.7 (C-2 and C-5), 109.0 (C-2' and C-2"), 113.9 (C-5' and C-5"), 119.3 (C-6' and C-6"), 132.5 (C-1' and C-1"), 144.3 (C-4 ' and C-4"), 146.2 (C-3' and C-3").
[0066] 3-2. Triasprosin (compound 2)
[0067] colorless oil; 1 H-NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com