Photoelectric functional aromatic phosphine oxide compound, and preparation method and application thereof
A technology of aromatic phosphine oxide and photoelectric function, applied in the field of photoelectric functional aromatic phosphine oxide compound and its preparation and application, can solve the problems of concentration quenching, poor efficiency stability, high driving voltage of electrophosphorescent device, etc., and achieve the goal of wide application prospect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0040] Specific implementation mode 1: This implementation mode is an aromatic phosphine oxide compound with photoelectric function, and its general structural formula is as follows:
[0041] Wherein, X is two hydrogens and Y is one hydrogen; or X is two hydrogens and Y is diphenylphosphine oxide; or X is isopropylidene and Y is diphenylphosphine oxide.
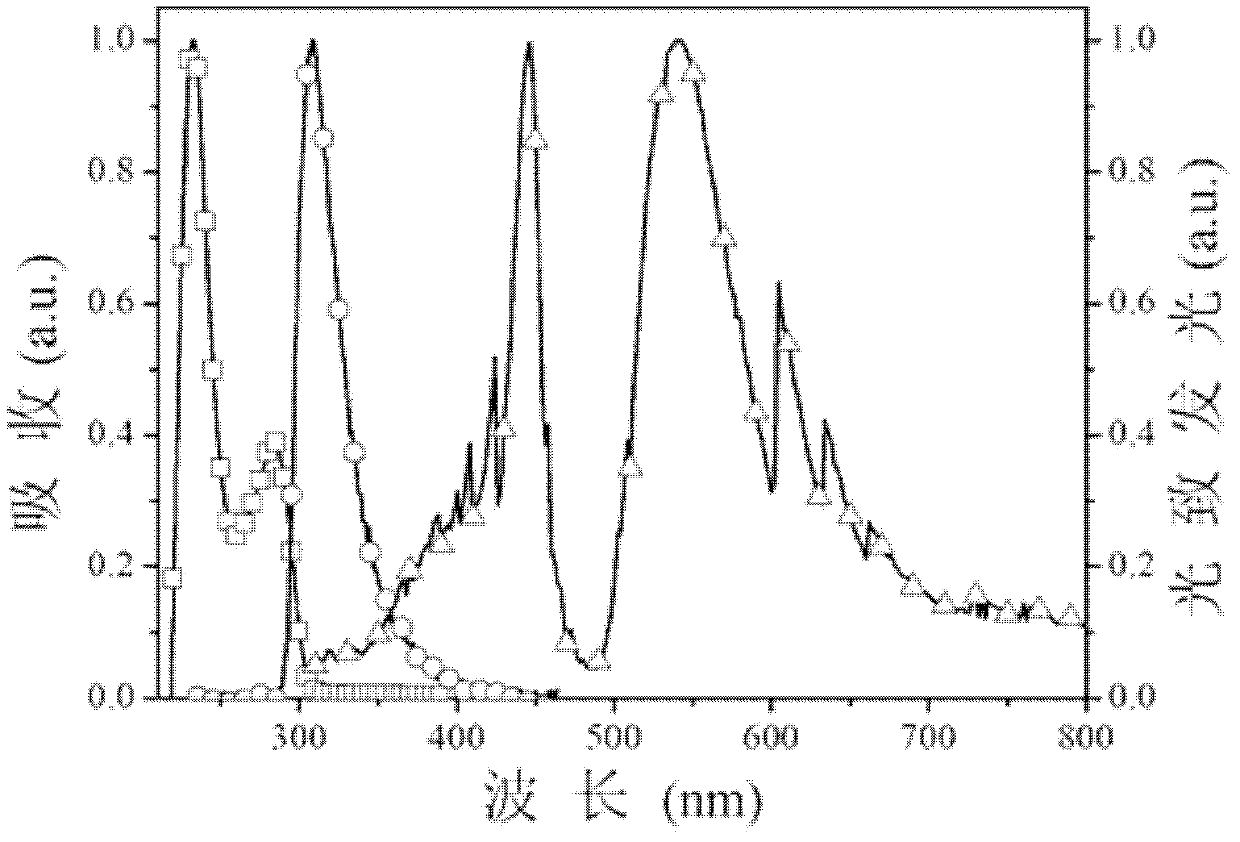
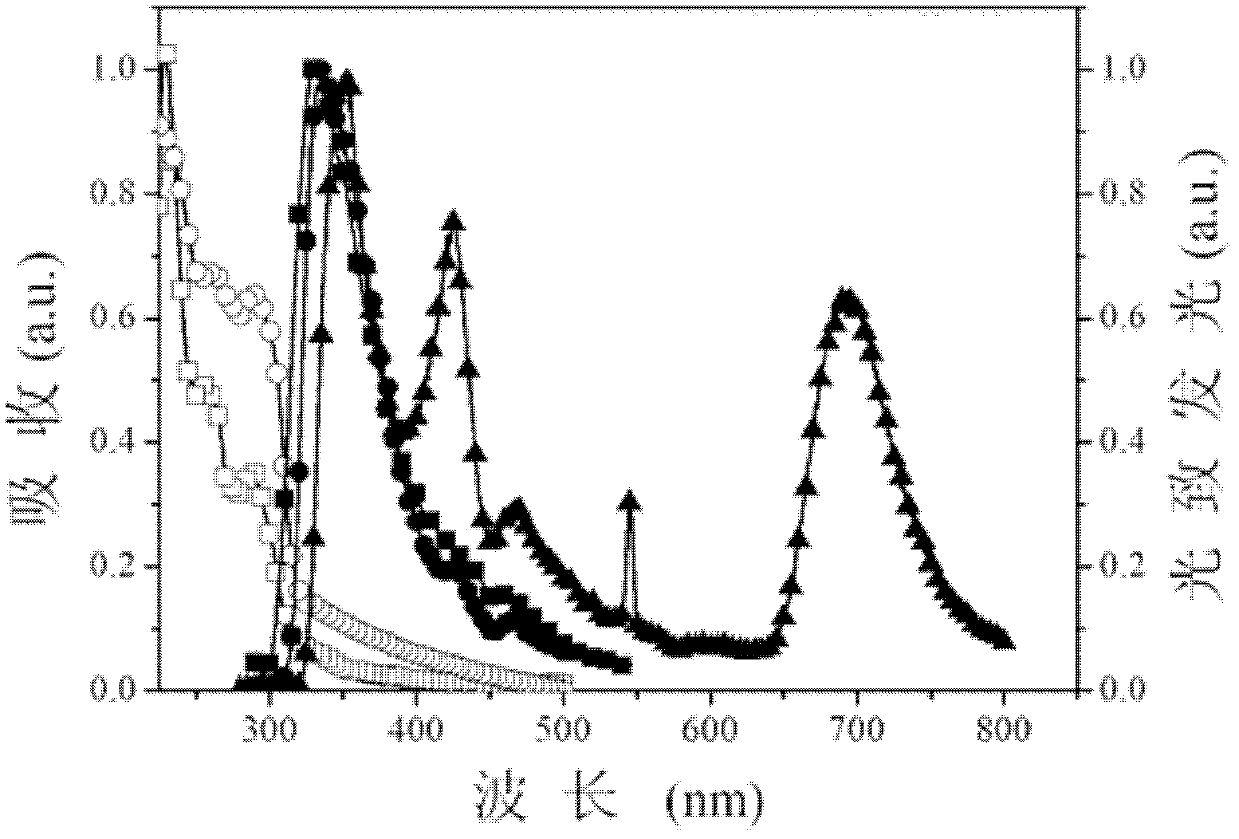
[0042] The three kinds of photoelectric functional aromatic phosphine oxide compounds in this embodiment have multiple conjugation breaking structures, and their molecules form multiple conjugating breaking systems through P=O, -O-, which are in tetramethylethylenediamine (TMEDA) In the presence of phenylene ether, the 2-position or 2,2'-position, or the 4,5-position of 9,9-dimethyl-xanthene undergoes lithiation, phosphine and oxidation reactions respectively.
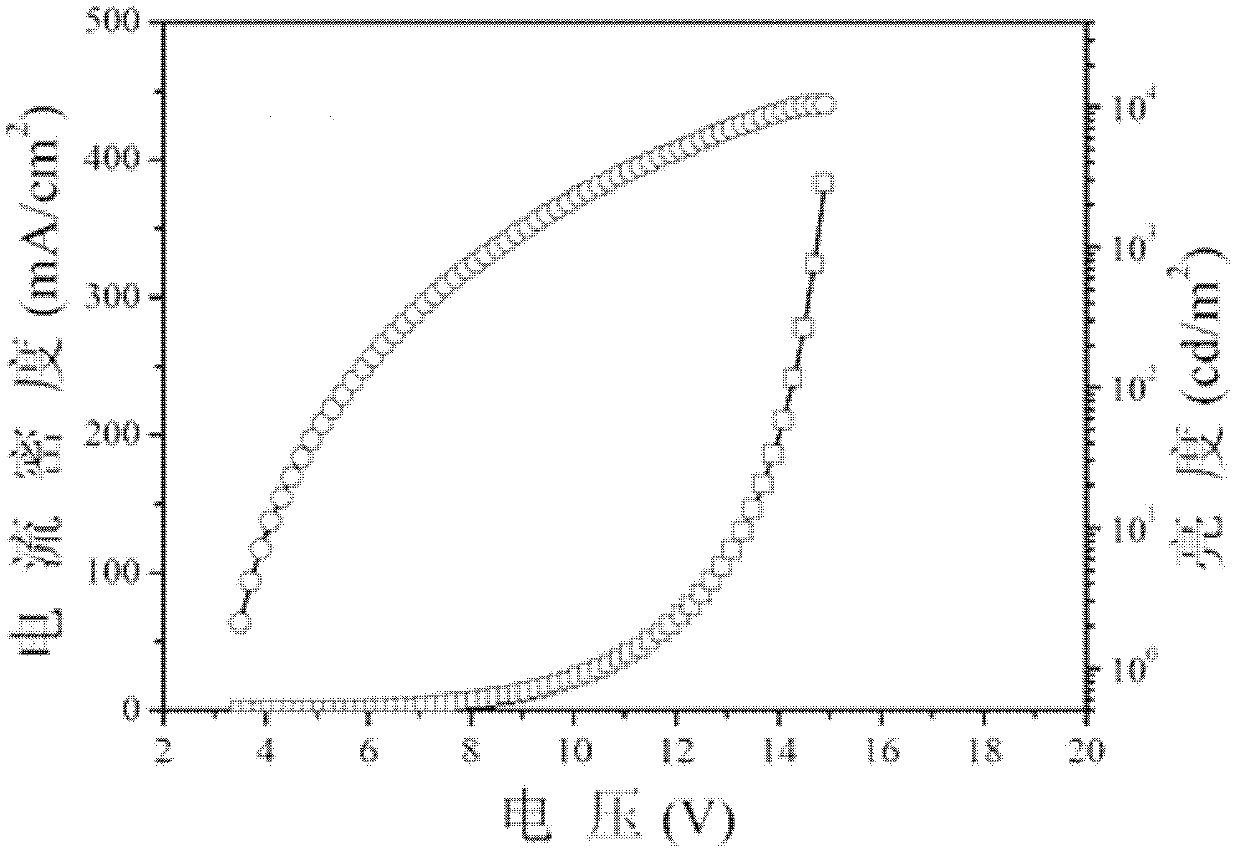
[0043] From the perspective of molecular design, it is necessary to fully consider the relationship between molecular structure and performance, and make a reasonab...
specific Embodiment approach 2
[0044] Specific embodiment two: the difference between this embodiment and specific embodiment one is that X is two hydrogens, Y is one hydrogen, and the structural formula is:
[0045] Other parameters are the same as in the first embodiment.
[0046] The photoelectric functional aromatic phosphine oxide compound of this embodiment is obtained by lithiation, phosphine and oxidation reactions at the 2-position of phenyl ether; it is abbreviated as DPESPO.
specific Embodiment approach 3
[0047] Specific embodiment three: the difference between this embodiment and specific embodiment one is that X is two hydrogens, Y is diphenylphosphine oxide, and the structural formula is:
[0048] Other parameters are the same as in the first embodiment.
[0049] The photoelectric functional aromatic phosphine oxide compound of this embodiment is obtained by lithiation, phosphination and oxidation reactions on the 2 and 2' positions of phenyl ether respectively; it is abbreviated as DPEPO.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap