Process for preparing cinacalcet
A technology of compounds and intermediates, applied in the field of preparation of active product component cinacalcet, which can solve problems such as difficult synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
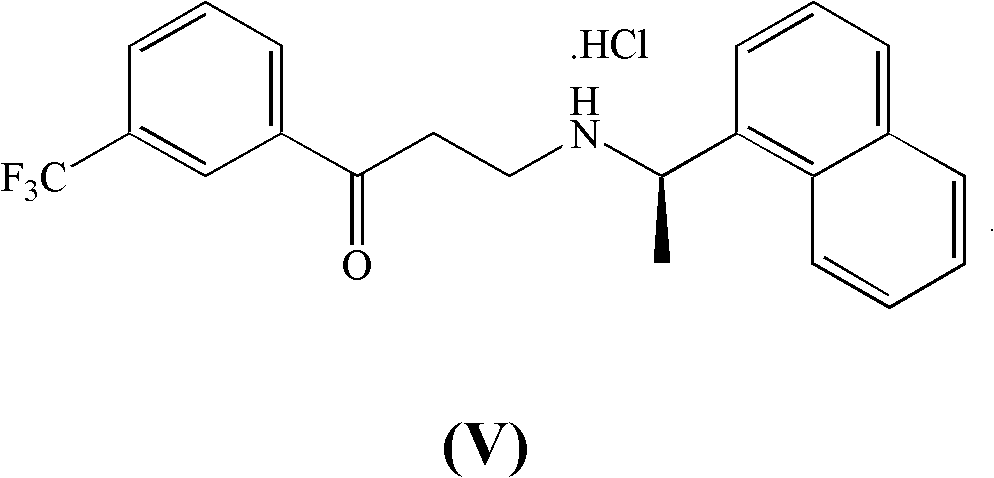
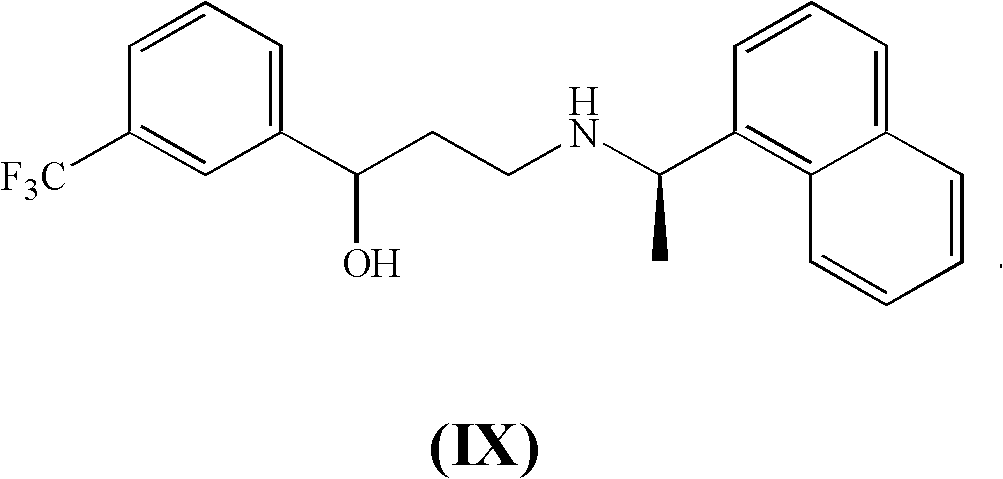
[0100] Synthesis of (R)-3-(1-(naphthalen-1-yl)ethylamino)-1-(3-(trifluoromethyl)phenyl)propan-1-ol hydrochloride (IXa)
[0101]
[0102] (R)-3-(1-(naphthalen-1-yl)ethylamino)-1-(3-(trifluoromethyl)phenyl)propan-1-one hydrochloride (V) (15.95g, 39.104mmol) was suspended in cold methanol (50ml) at -10°C, followed by the slow addition of sodium borohydride solution (0.75g, 19.610mmol), 30% w / w aqueous sodium hydroxide (5.74g, 43.014mmol) and water (5ml) in order to keep the internal temperature below 0°C. The reaction mixture was stirred at 0°C for 0.5 hours and then quenched by the addition of 30% w / w aqueous hydrochloric acid to pH = 1, then water (40ml) and allowed to reach room temperature. The thick suspension thus formed was heated to 50°C, stirred for 20 minutes, then cooled to 5°C. The precipitate was filtered, washed with a 9:1 vol / vol water / methanol mixture (10 ml) and dried under vacuum at 50°C. Obtained 14.69 g (35.841 mmol) of high quality (R)-3-(1-(naphthalene...
Embodiment 2
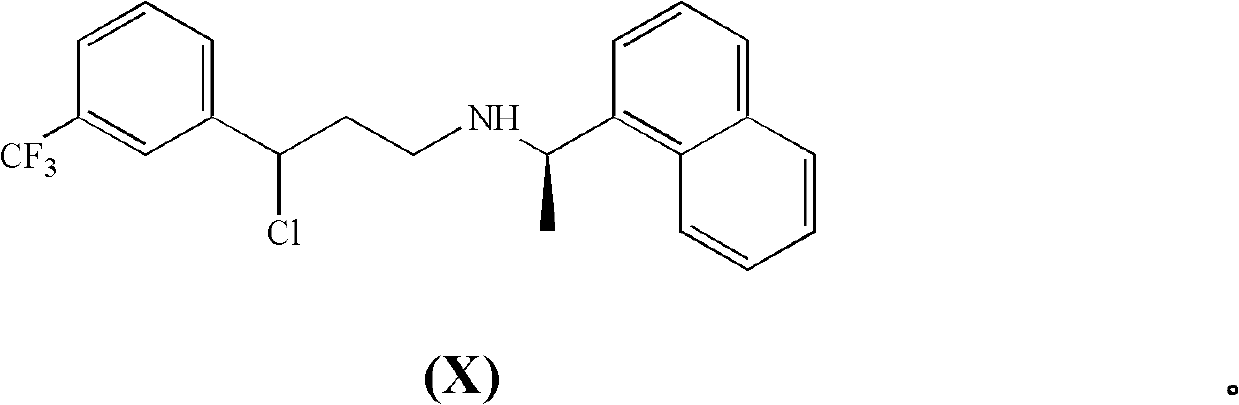
[0104] (R)-3-Chloro-N-(1-(naphthalene-1-yl)ethyl)-3-(3-(trifluoromethyl)phenyl)propan-1-amine hydrochloride (X) synthesis
[0105]
[0106] (R)-3-(1-(naphthalen-1-yl)ethylamino)-1-(3-(trifluoromethyl)phenyl)propan-1-ol hydrochloride (IXa) (20.0 g, 48.796mmol) was suspended in toluene (140ml) at 40°C, and phosphorus oxychloride (4.3g, 28.044mmol) was added dropwise within 10 minutes. The reaction mixture was stirred at 60°C for 2 hours, then DMF (1.0 g) was added at 40°C, followed by additional phosphorus oxychloride (3.2 g, 20.870 mmol). The mixture was stirred overnight at 40°C, then MTBE (40ml) was added. Volatiles were removed and MTBE was recovered by repeated distillation under vacuum. Thereafter, a 1:1 vol / vol toluene / MTBE solution heated to 70°C was added and allowed to cool slowly to 15°C-20°C. The precipitate thus obtained was aged overnight at room temperature, then filtered and washed with a 1:1 vol / vol toluene / MTBE mixture (3 x 12 ml). After drying under va...
Embodiment 3
[0108] (R)-3-Chloro-N-(1-(naphthalene-1-yl)ethyl)-3-(3-(trifluoromethyl)phenyl)propan-1-amine hydrochloride (X) and (R, E)-N-(1-(naphthalene-1-yl)ethyl)-3-(3-(trifluoromethyl)phenyl)prop-2-en-1-amine hydrochloride (XI )Synthesis
[0109]
[0110] Method A
[0111] (R)-3-(1-(naphthalen-1-yl)ethylamino)-1-(3-(trifluoromethyl)phenyl)propan-1-ol hydrochloride (IX) (35.0g, 85.393mmol) was suspended in toluene (150ml) at 20°C, and thionyl chloride (11.2.g, 94.141mmol) was added slowly. The reaction mixture was stirred at 30°C-40°C for 4-5 hours, then the solvent was distilled off under vacuum. After several distillation / refill cycles, the residual toluene slurry was rinsed with isopropanol. The resulting isopropanol solution was refluxed for 1 hour, then cooled to 45°C and methyl tert-butyl ether (MTBE) (70ml) was added. The suspension thus obtained was stirred at 45°C for 1 hour, then cooled to 0°C and aged for 1 hour. After filtration, washing with a 3:1 vol / vol isopropan...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap