Modification method of high-rate lithium-rich cathode material
A lithium-rich positive electrode material and high-rate technology, applied in battery electrodes, electrical components, circuits, etc., can solve the problems that cannot meet the needs of fast charging of electric equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. According to the molecular formula Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 The ratio of Li, Ni, Mn to prepare LiNO 3 , Ni(NO 3 ) 2 and Mn(NO 3 ) 2 Mixed solution, the total metal ion concentration is 1mol / L;
[0023] 2. Configure the citric acid solution according to the ratio of citric acid: total metal ions = 1:1 (molar ratio). Under strong stirring, add the mixed solution of the metal salt dropwise to the citric acid solution, stir and heat in a water bath at 80°C until Yellow-green gel, dry and slightly fluffy bulk precursor after drying in a vacuum oven at 150°C for 12 hours;
[0024]3. Heat the precursor obtained above at 480°C for 10 hours in an air atmosphere, then heat it up to 750°C for 15 hours, and cool it to room temperature with the furnace to obtain the lithium-ion battery cathode material Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 .
[0025] 4. Disperse the obtained lithium-rich cathode material in 0.284g / L NH 4 h 2 PO 4 Sonicate the solution for 1 hour, then ...
Embodiment 2
[0030] 1-3 steps are the same as embodiment 1;
[0031] 4. Disperse the lithium-rich cathode material prepared above in 0.426g / L NH 4 h 2 PO 4 Sonicate the solution for 1 hour, then stir vigorously for 2 hours, and drop NH with a concentration of 0.434 g / L into it 4 VO 3 solution, stirred for 1 hour, evaporated to dryness in a water bath at 80°C, and dried at 80°C;
[0032] 5. Then sinter at 400°C for 6 hours to obtain surface-modified Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 .
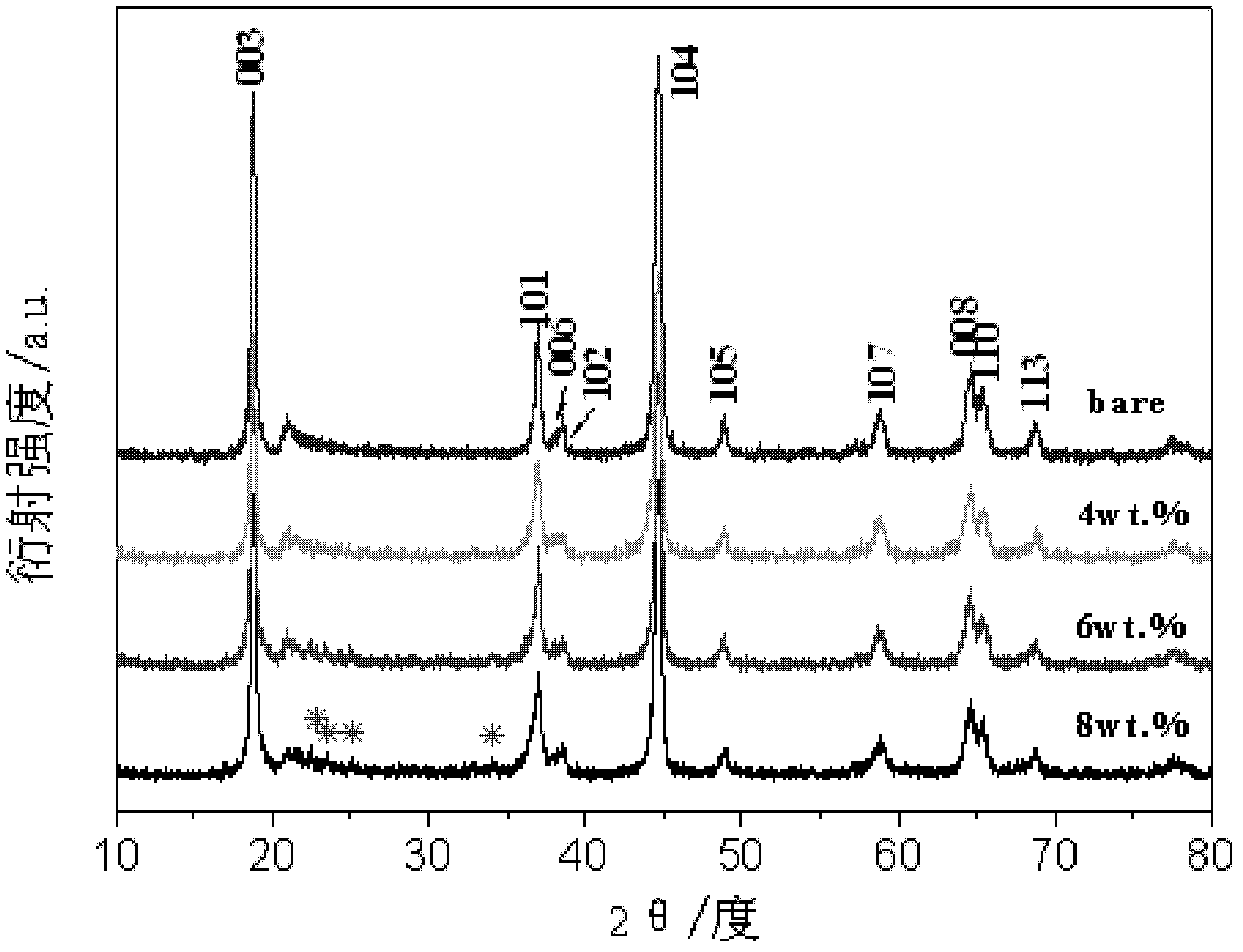
[0033] X-ray diffraction (XRD) analysis showed that the main phase of the product was Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 ( figure 1 The spectrum corresponding to 6wt.% of the middle coating rate), only Li appeared between 20-35° 3 PO 4 The miscellaneous peaks (marked with an asterisk), whose structure has not been destroyed.
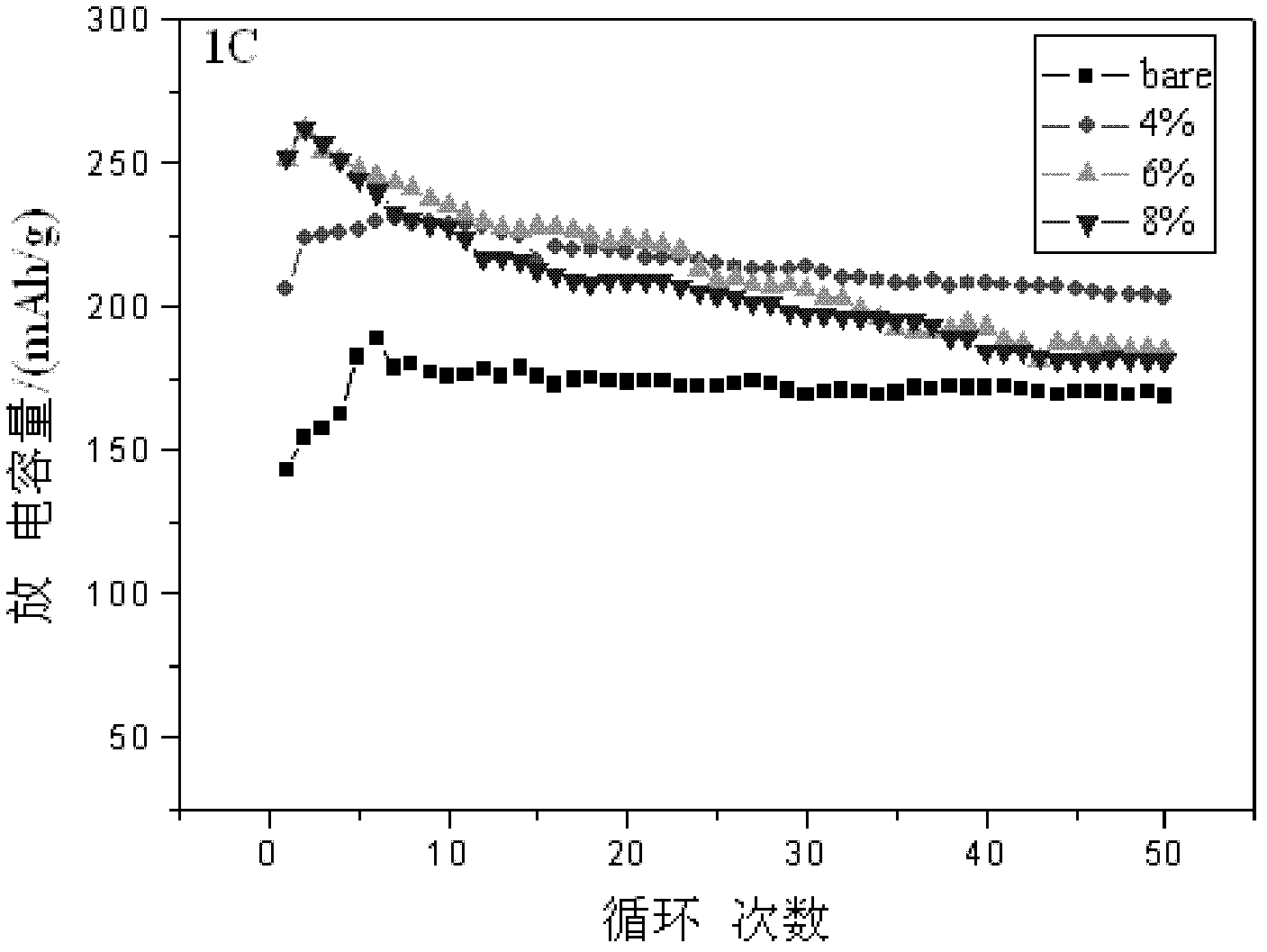
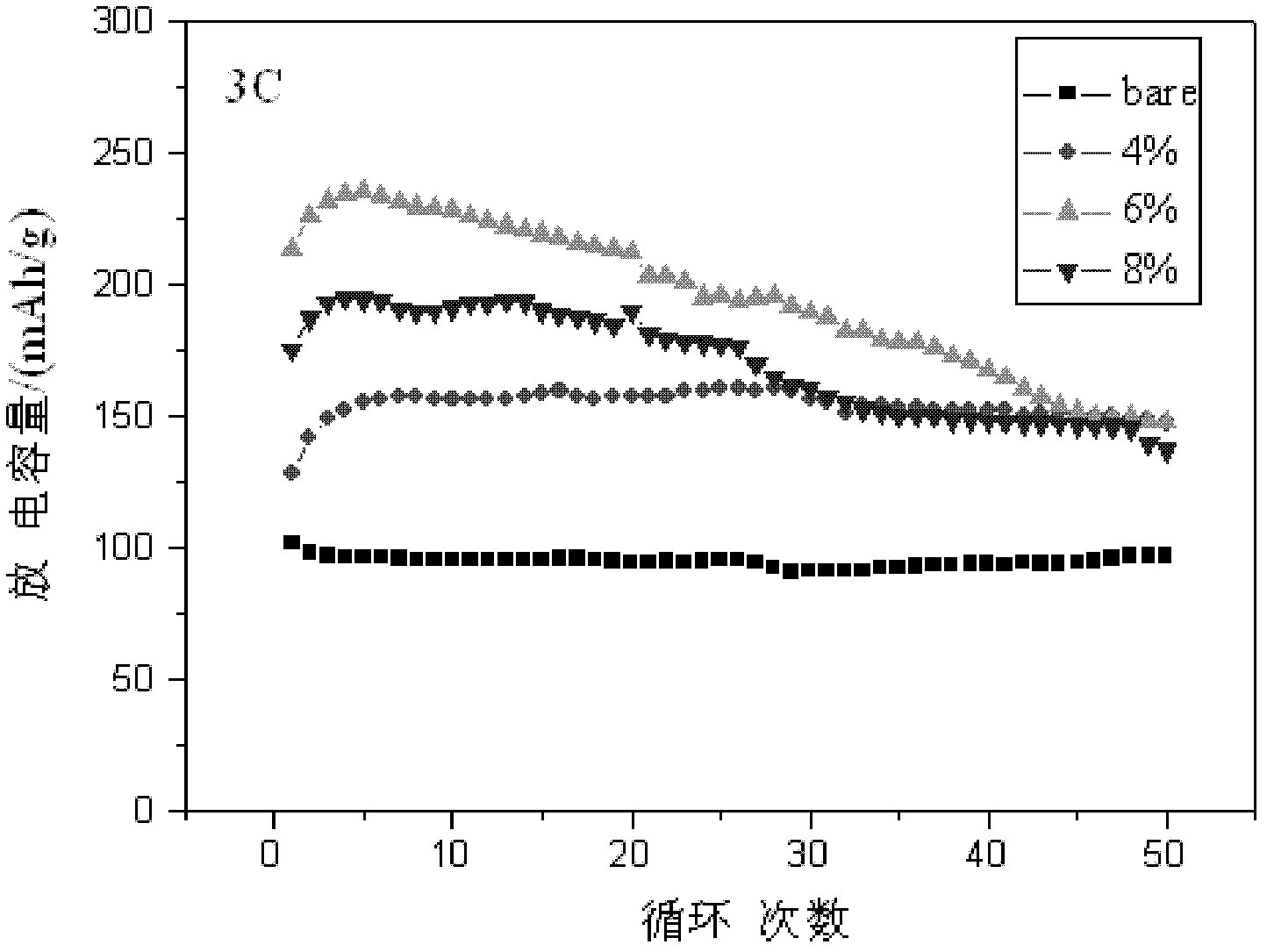
[0034] Electrochemical tests show that when charging and discharging at 1C and 3C, the initial discharge capacity is 250mAh / g (see figure 2 6wt.% corresponding graph) and 213mAh...
Embodiment 3
[0036] 1-3 steps are the same as embodiment 1;
[0037] 4. Disperse the obtained lithium-rich cathode material in 0.568g / L NH 4 h 2 PO 4 Sonicate the solution for 1 hour, then stir vigorously for 2 hours, and drop NH with a concentration of 0.578 g / L into it 4 VO 3 solution, stirred for 1 hour, evaporated to dryness in a water bath at 80°C, and dried at 80°C;
[0038] 5. Then sinter at 400°C for 6 hours to obtain surface-modified Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 .
[0039] X-ray diffraction (XRD) analysis showed that the main phase of the product was Li[Ni 0.2 Li 0.2 mn 0.6 ]O 2 ( figure 1 The spectrum corresponding to 8wt.% of the medium coating rate), only Li appears between 20-35° 3 PO 4 The miscellaneous peaks (marked with an asterisk), whose structure has not been destroyed.
[0040] Electrochemical tests show that when charging and discharging at 1C and 3C, the first discharge capacity is 252mAh / g (see figure 2 Covering rate 8wt.% corresponding graph) and 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com