Preparing technology of erlotinib hydrochloride
A technology for the preparation of erlotinib hydrochloride, which is applied in the field of preparation of pharmaceutical compounds, can solve problems such as unfavorable post-processing and large environmental pollution, and achieve the effects of short reaction process route, simple and easy operation, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0028] Example 1
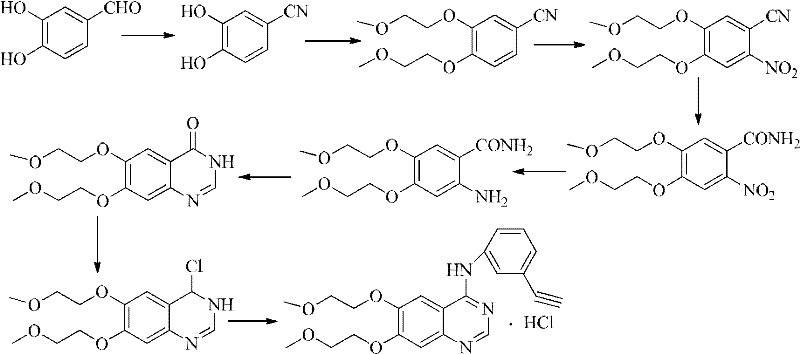
[0029] In the preparation process of erlotinib hydrochloride, 2-nitro-4,5-bis(2-methoxyethoxy)benzonitrile is used as the starting material to obtain 2-amino-4 through nitro reduction, 5-bis(2-methoxyethoxy)benzonitrile is prepared according to process route (1), which specifically includes the following steps:
[0030] 1. Preparation of 2-amino-4,5-bis(2-methoxyethoxy)benzonitrile
[0031] Mix 2-nitro-4,5-bis(2-methoxyethoxy)benzonitrile (100g, 0.336mol), sodium dithionite (204g, 1.176mol) and water (1.7L), stir and heat to 50~55℃, continue to stir for 2 hours, quickly raise the temperature to 65~70℃, slowly add concentrated hydrochloric acid (450ml) dropwise, the temperature of the reaction solution drops to room temperature after the dropwise addition, adjust the pH value with 40% sodium hydroxide solution To 10, dichloromethane (3×75ml) extraction, the combined organic phase was washed with purified water (2×50ml), saturated brine (1×50ml), and the solvent wa...
Example Embodiment
[0036] Example 2
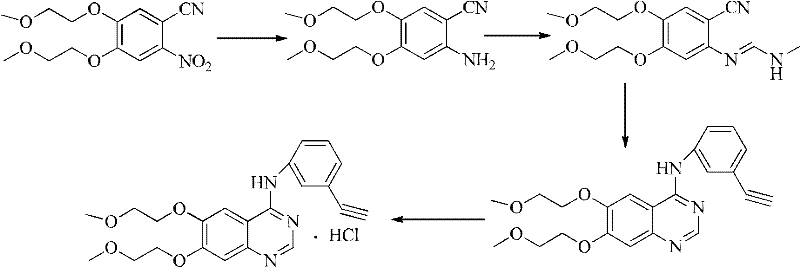
[0037] In the preparation process of erlotinib hydrochloride, 2-nitro-4,5-bis(2-methoxyethoxy)benzonitrile is used as the starting material to obtain 2-amino-4 through nitro reduction, 5-bis(2-methoxyethoxy)benzonitrile is prepared according to process route (2), which specifically includes the following steps:
[0038] 1. Preparation of 2-amino-4,5-bis(2-methoxyethoxy)benzonitrile
[0039] The operation was the same as that of step 1 in Example 1, to obtain a brown-yellow solid (84.65 g, 94.06%).
[0040] 2. Preparation of Schiff base of 3-ethynylaniline
[0041] Add 3-ethynylaniline (32.6, 0.28mol), toluene (800ml), acetic acid (0.13ml) and DMF-DMA (60g, 0.505mol) into a reaction flask equipped with an oil-water separator, mix and stir, and heat to reflux 3. After the reaction was completed, the solvent was evaporated to obtain an oily liquid (37.1 g, 0.257 mol). The next step was carried out without purification.
[0042] 3. Preparation of [6,7-bis(2-methoxyethox...
Example Embodiment
[0047] Example 3
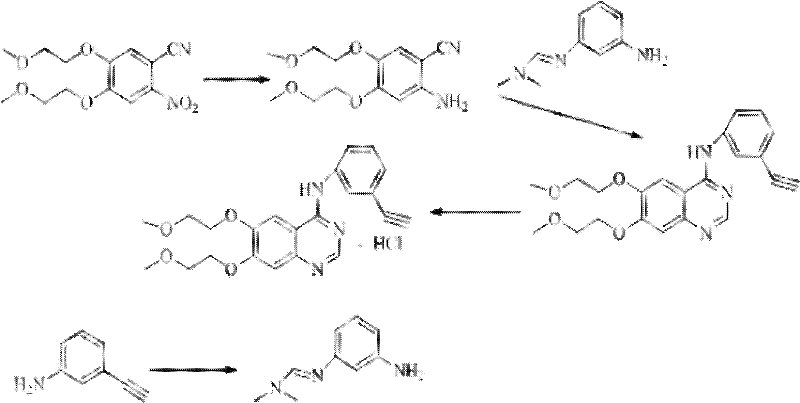
[0048] In the preparation process of erlotinib hydrochloride, 2-nitro-4,5-bis(2-methoxyethoxy)benzonitrile is used as the starting material to obtain 2-amino-4 through nitro reduction, 5-bis(2-methoxyethoxy)benzonitrile is prepared according to process route (1), which specifically includes the following steps:
[0049] 1. Preparation of 2-amino-4,5-bis(2-methoxyethoxy)benzonitrile
[0050] Mix 2-nitro-4,5-bis(2-methoxyethoxy)benzonitrile (100g, 0.336mol), iron powder (65.86g, 1.176mol) and water (1.7L), stir and heat to 50~55℃, continue to stir for 2 hours, quickly raise the temperature to 65~70℃, slowly add concentrated hydrochloric acid (450ml) dropwise, the temperature of the reaction solution drops to room temperature after the dropwise addition, adjust the pH value with 40% sodium hydroxide solution To 10, dichloromethane (3×75ml) extraction, the combined organic phase was washed with purified water (2×50ml), saturated brine (1×50ml), and the solvent was ev...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap