Unit dose formulation of antidotes for factor Xa inhibitors and methods of using the same
A unit dose, inhibitor technology, applied in the direction of anti-toxic agents, biochemical equipment and methods, medical preparations containing active ingredients, etc., can solve problems such as low procoagulant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
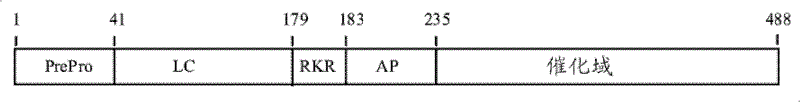
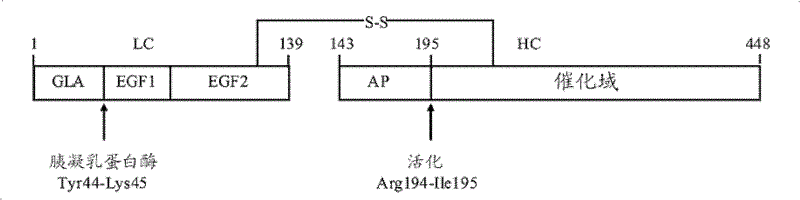
[0368] Example 1. Preparation of Gla-depleted anhydro-fXa by chymotrypsin digestion
[0369] According to Morita (Morita, T.) et al., Journal of Biochemistry (J.Bio.Chem.), 1986,261 (9): 4015-4023 program by anhydrous-fXa (wherein dehydroalanine De-Gla anhydrous-fXa was prepared by incubating with chymotrypsin in 0.05M Tris-HCl, 0.1M NaCl at pH 7.5 and 22°C for 60 minutes. In a typical experimental setup, 0.5 milligram per milliliter (mg / mL) anhydrous-fXa was incubated with 5 units / milliliter (U / mL) α-chymotrypsin-agarose beads under gentle agitation. At the end of the reaction, remove the α-chymotrypsin-agarose beads by centrifugation or filtration. This was subsequently combined with the excess inhibitors 4-amidino-phenyl-methane-sulfonyl fluoride (APMSF), tosyl-L-lysine chloromethyl ketone (TLCK), and tosyl-L- Phenylalanine chloromethyl ketone (TPCK) was incubated with to quench residual fXa activity or any chymotrypsin activity that might be leached from the beads....
Embodiment 2
[0373] Example 2. Analysis of Thrombin Generation in Platelet Poor Plasma (PPP) or Platelet Rich Plasma (PRP)
[0374] In this example, human platelet-poor or platelet-rich plasma samples were prepared from blood of healthy donors drawn into 0.32% citrate. PRP and PPP were prepared by spinning anticoagulated blood at about 100X gravity or 1000X gravity, respectively, for 20 minutes at room temperature. Mix 75-100 microliters (uL) of plasma with CaCl 2 Mix with Z-Gly-Gly-Arg-aminomethylcoumarin (Z-GGR-AMC, fluorogenic substrate for thrombin). Tissue factor (Innovin, Dade Behring) was added to initiate thrombin generation. For a typical experiment, the reaction mixture contained 15 millimolar (mM) Ca 2+ , 100 micromolar (μM) Z-GGR-AMC, and 0.1 nanomolar (nM) tissue factor (TF) (Innovin). Thrombin formation was monitored continuously at 37°C by a fluorescent plate reader (Molecular Devices) measuring relative fluorescence units (RFU). When present, inhibitors and antidotes w...
Embodiment 3
[0376] Example 3. Coagulation Prolongation Analysis
[0377] Two coagulation assay formats were used to test the effect of factor Xa inhibitors and antidotes on prolonged coagulation. In the first format, a 96-well plate is used to measure multiple samples simultaneously. In a second assay format, aPTT was measured using a conventional coagulation instrument (MLA Electra 800 Automatic Coagulation Timer).
[0378] In the 96-well plate format method, human platelet-poor plasma or platelet-rich plasma was prepared in a procedure similar to that in Example 2. Utilize 75-100uL of plasma with CaCl 2 Recalcify, incubate at 37°C for 3 minutes and remove calcification by adding tissue factor (Innovin, Dade Behring) or aPTT reagent (Actin FS, Dade Behring) ) to initiate blood clot formation. Changes in OD405 were continuously monitored by a plate reader (Molecular Devices). Coagulation time was defined as the time (in seconds) when the half-maximum change in absorbance (OD405nm) wa...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap