GLP-1 analogs, preparation method thereof application thereof
A technology of GLP-1 and analogues, which is applied in peptide preparation methods, chemical instruments and methods, drug combinations, etc., can solve problems such as failure to meet clinical standards, inconvenient clinical use, and short half-life of liraglutide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Example 1: Solid Phase Synthesis of Polypeptides
[0157] The synthesis of the polypeptides of the present invention was carried out using the solid-phase polypeptide synthesis method of the Fmoc strategy, using a CS 336X instrument produced by CSBio. The method of synthesis was carried out according to the manufacturer's instrument instructions.
[0158] The prepared polypeptide was purified by HPLC C18 semi-preparative column, and the mobile phase was acetonitrile. After desalting and freeze-drying, polypeptide freeze-dried powder is obtained. The polypeptides contained in this patent all contain disulfide bonds, and ammonium bicarbonate or other reducing agents are used to form the disulfide bonds in the polypeptides.
Embodiment 2
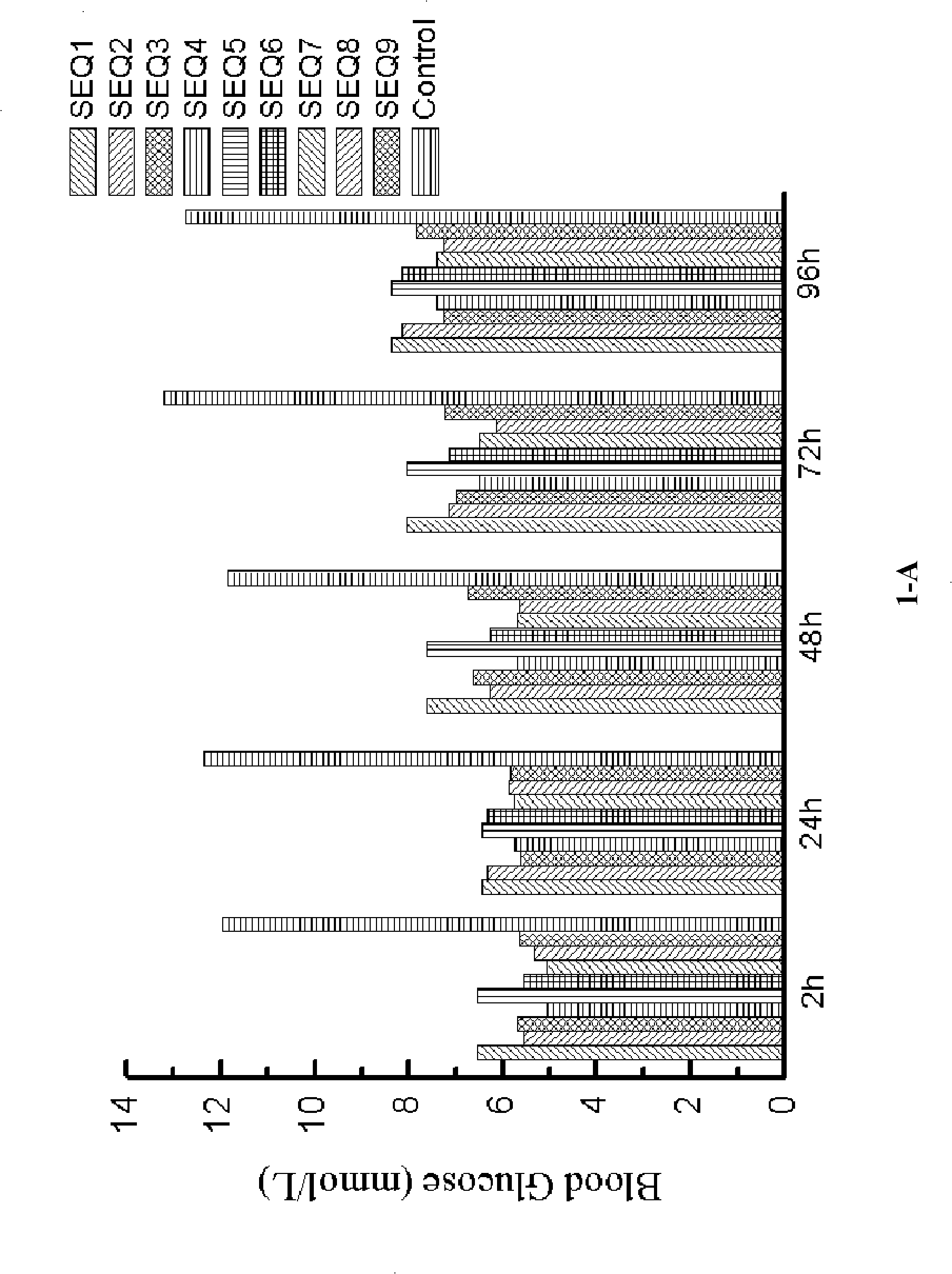
[0159] Example 2: Hypoglycemic function associated with GLP-1 analogs (formula II)
[0160] In this example, the polypeptides used are as follows:
[0161] SEQ ID NO 1: 7 HAEGT FTSCV SSYLE GQAAK EFIAW LVKGR G 37 GGGGGC GGG
[0162] SEQ ID NO 2: 7 HAEGT FTSCV SSYLE GQAAK EFIAW LVKGR G 37 GGGGGGGGGGC GGG
[0163] SEQ ID NO 3: 7 HAEGT FTSCV SSYLE GQAAK EFIAW LVKGR G 37 GGGGGGGGGGGGGGGC GGG
[0164] SEQ ID NO 4: 7 HAEGT FTSCV SSYLE GQAAK EFIAW LVKGR G 37 GGGGGC GGGGG G
[0165] SEQ ID NO 5: 7 HAEGT FTSCV SSYLE GQAAK EFIAW LVKGR G 37 GGGGGC GGGGG GGGG
[0166] SEQ ID NO 6: 7 HAEGT FTSCV SSYLE GQAAK EFIAW LVKGR G 37 GGGGGGGGGGC GGGGG G
[0167] SEQ ID NO 7: 7 HAEGT FTSCV SSYLE GQAAK EFIAW LVKGR G 37 GGGGGGGGGGC GGGGG GGGG
[0168] SEQ ID NO 8: 7 HAEGT FTSCV SSYLE GQAAK EFIAW LVKGR G 37 GGGGGGGGGGGGGGGC GGGGG G
[0169] SEQ ID NO 9: 7 HAEGT FTSCV SSYLE GQAAK EFIAW LVKGR G 37 GGGGGGGGGGGGGGGC GGGGG GGGG
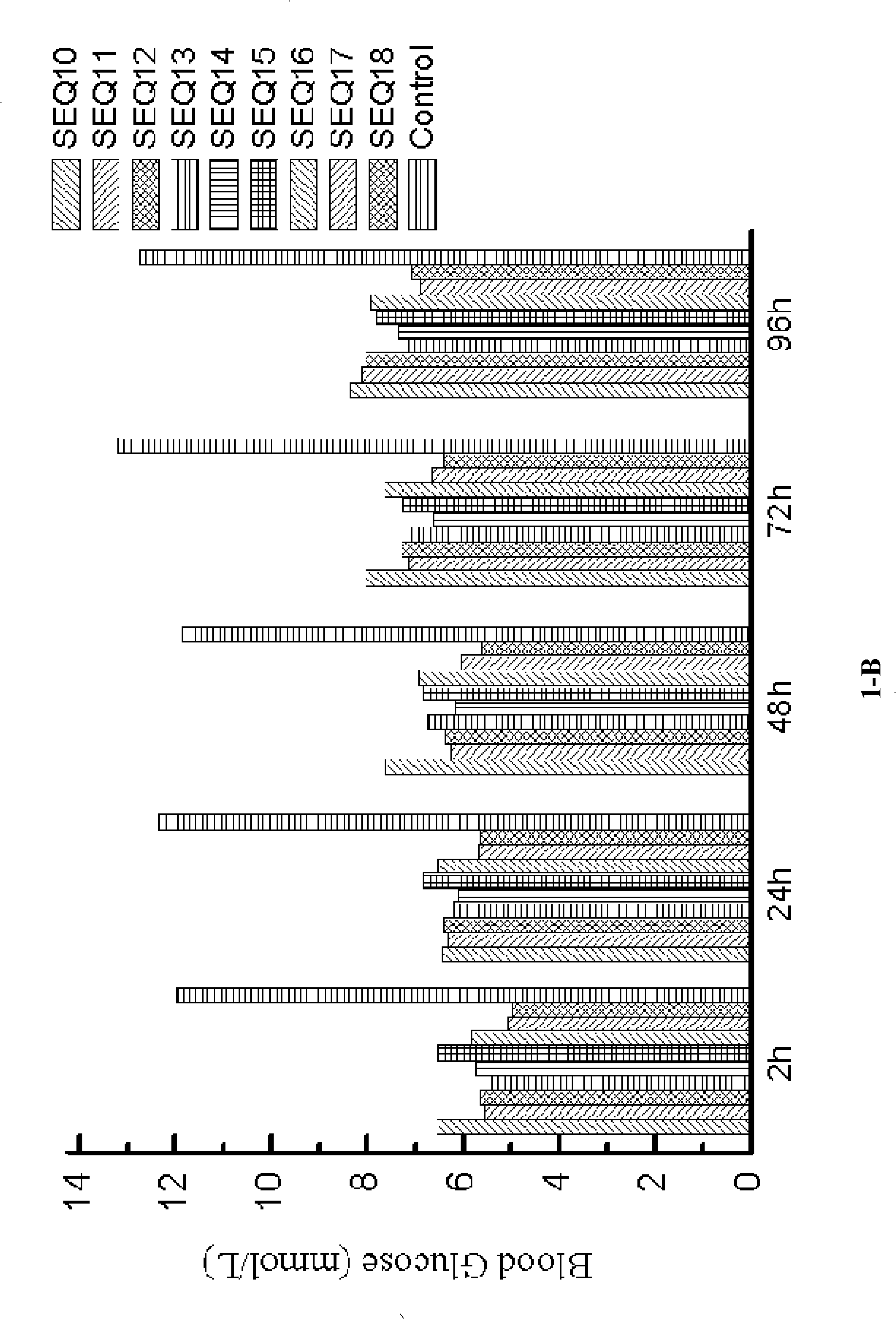
[0170] SEQ ID NO 10: 7 HAEGT FTSCV SSYLE GQAAK EFIAW...
Embodiment 3
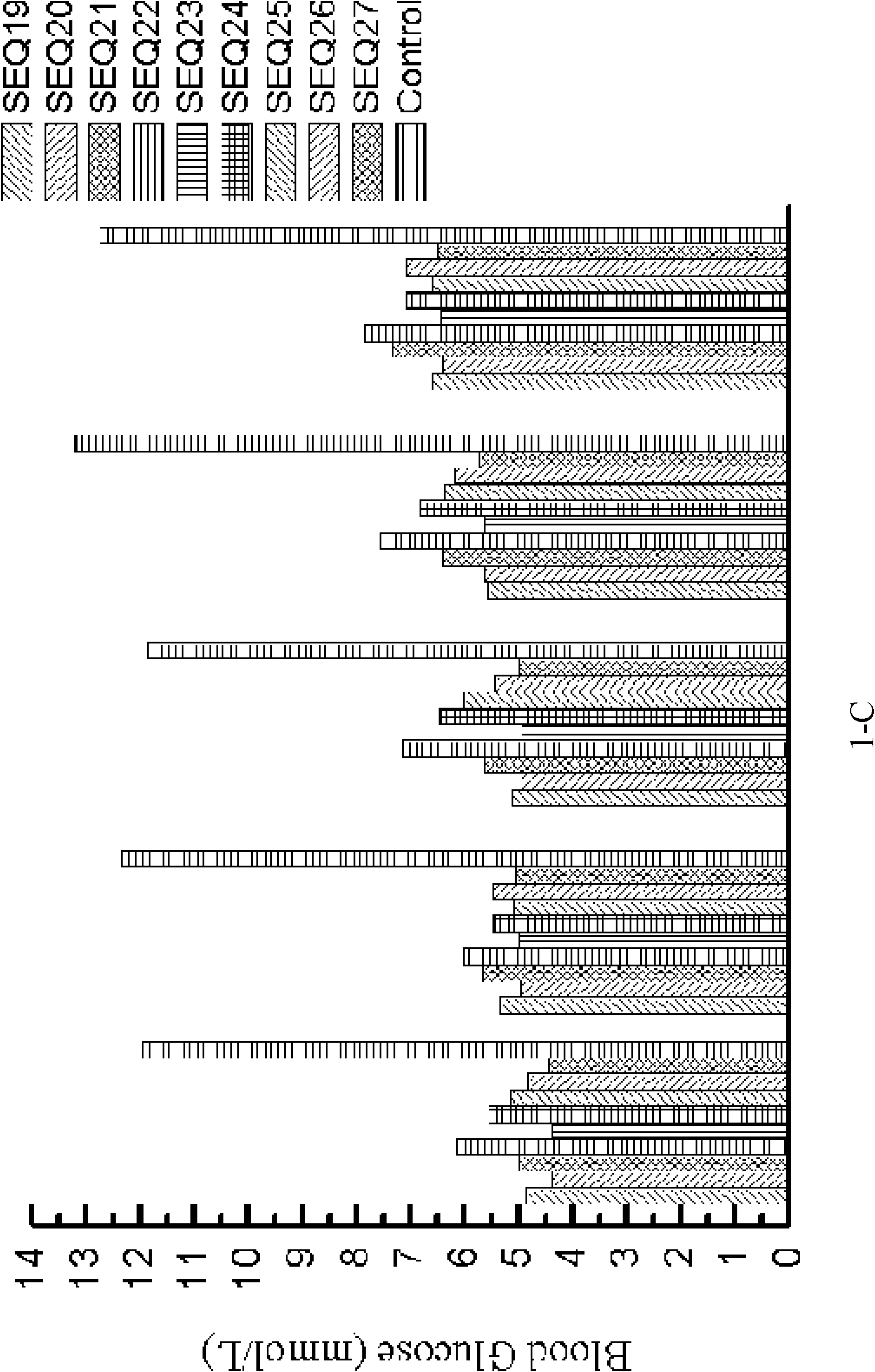
[0189] Example 3: Hypoglycemic function associated with GLP-1 analogs (formula III).
[0190]In this example, the polypeptides used are as follows:
[0191] SEQ ID NO 28: 7 HAEGT FTSDV SSYLE GQAAK EFICW LVKGR G 37 G CGGGGG GGGGG
[0192] SEQ ID NO 29: 7 HAEGT FTSDV SSYLE GQAAK EFICW LVKGR G 37 G CGGGGG GGGGGGGGGG
[0193] SEQ ID NO 30: 7 HAEGT FTSDV SSYLE GQAAK EFICW LVKGR G 37 G CGGGGG GGGGGGGGGGGGGGGGGGGG
[0194] SEQ ID NO 31: 7 HAEGT FTSDV SSYLE GQAAK EFICW LVKGR G 37 GG CGGGGG GGGGG
[0195] SEQ ID NO 32: 7 HAEGT FTSDV SSYLE GQAAK EFICW LVKGR G 37 GG CGGGGG GGGGGGGGGG
[0196] SEQ ID NO 33: 7 HAEGT FTSDV SSYLE GQAAK EFICW LVKGR G 37 GG CGGGGG GGGGGGGGGGGGGGG
[0197] SEQ ID NO 34: 7 HAEGT FTSDV SSYLE GQAAK EFICW LVKGRG 37 GGGGG C GGGGG GGGGG
[0198] SEQ ID NO 35: 7 HAEGT FTSDV SSYLE GQAAK EFICW LVKGRG 37 GGGGG C GGGGG GGGGGGGGGG
[0199] SEQ ID NO 36: 7 HAEGT FTSDV SSYLE GQAAK EFICW LVKGRG 37 GGGGG C GGGGG GGGGGGGGGGGGGGG
[0200] SEQ ID NO 37:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com