Bipolar blue-light main material and preparation method thereof and organic electroluminescent device
A blue-light host material, bipolar technology, applied in the field of copolymers, can solve the problem of lack of charge transport performance, achieve high triplet energy level, good electron injection and hole blocking and exciton blocking capabilities, excellent thermal stability performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The preparation scheme of the above-mentioned bipolar blue light host material is as follows:
[0021] Step S1, providing the structural formula as The 2,7-dibromofluorenone and the structural formula are Triphenylamine derivatives; in the formula, R is H or C 1 ~C 12 The alkyl group, preferably R is C 1 ~C 12 straight-chain or branched-chain alkyl;
[0022] Step S2: In an oxygen-free environment (composed of inert gas, such as nitrogen, argon, etc.), 2,7-dibromofluorenone, triphenylamine derivatives and methanesulfonic acid ( CH 3 SO 3 H, used in acidic environment) is placed in the reactor, stirred and reacted at 140°C for 6h and then cooled to room temperature to obtain the structural formula: 9,9-bis(triphenylamino)-2,7-dibromofluorene derivative; the reaction formula is as follows:
[0023]
[0024] Step S3: Dissolve the 9,9-bis(triphenylamino)-2,7-dibromofluorene derivative prepared in step S2 in an organic solvent, and then add n-butyllithium (n -...
Embodiment 1
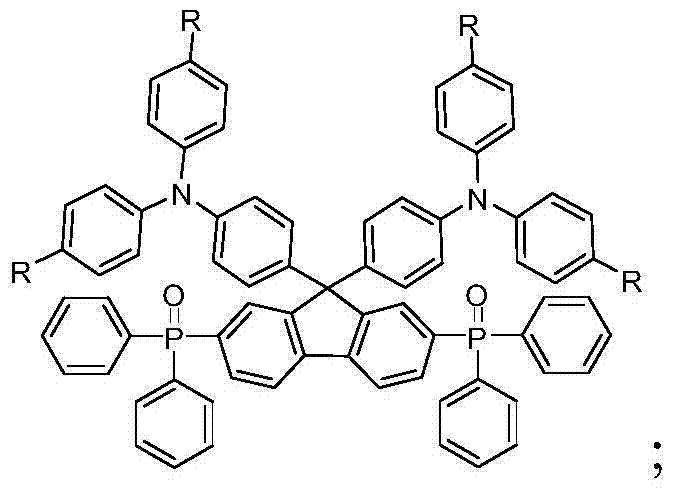
[0037] This embodiment discloses 2,7-bis(diphenylphosphinooxy)-9,9-bis(triphenylamino)fluorene (R is H) with the following structural formula:
[0038]
[0039] The preparation of the above-mentioned target molecule is as follows:
[0040] 1. 9,9-bis(triphenylamino)-2,7-dibromofluorene
[0041]
[0042] Under the protection of argon, 2,7-dibromofluorenone (3.38g, 10mmol), triphenylamine (34.3g, 140mmol) and methanesulfonic acid (0.96g, 10mmol) were respectively added into a 100mL three-necked flask. Stir the reaction at 140°C for 6h, stop the reaction and cool to room temperature, extract with dichloromethane, and wash with saturated sodium bicarbonate solution and distilled water respectively. The organic layer was concentrated to give a blue solid. The crude product was separated and purified by silica gel chromatography using petroleum ether / dichloromethane (3:1) as the eluent, and then recrystallized with acetone to obtain white 9,9-bis(triphenylamino)-2,7- Dibromof...
Embodiment 2
[0048] This embodiment discloses 2,7-bis(diphenylphosphinooxy)-9,9-bis[4-(di-p-propylphenyl)aminophenyl]fluorene (R is propyl) with the following structural formula:
[0049]
[0050] The preparation of the above-mentioned target molecule is as follows:
[0051] 1. 9,9-bis[4-(di-p-propylphenyl)aminophenyl]-2,7-dibromofluorene
[0052]
[0053] Under argon protection, in a 100mL three-necked flask, were added 2,7-dibromofluorenone (3.303g, 9mmol), 4-(di-p-propylphenyl)aniline (41.45g, 126mmol), methyl Sulfonic acid (0.9 g, 9 mmol). Stir the reaction at 140°C for 6h, stop the reaction and cool to room temperature, extract with dichloromethane, and wash with saturated sodium bicarbonate solution and distilled water respectively. The organic layer was concentrated to give a blue solid. The crude product was separated and purified by silica gel chromatography using petroleum ether / dichloromethane (3:1) as eluent, and then recrystallized with acetone to obtain a white solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com