High-heat resistant aliphatic polycarbonate based on 1,4:3,6-dianhydro-hexanehexol, and preparation method and application thereof
A technology of polycarbonate and hexanol, which is used in medical science, surgery, etc., can solve the problems of difficulty in removing by-product phenol, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1, copolymer shown in preparation formula I
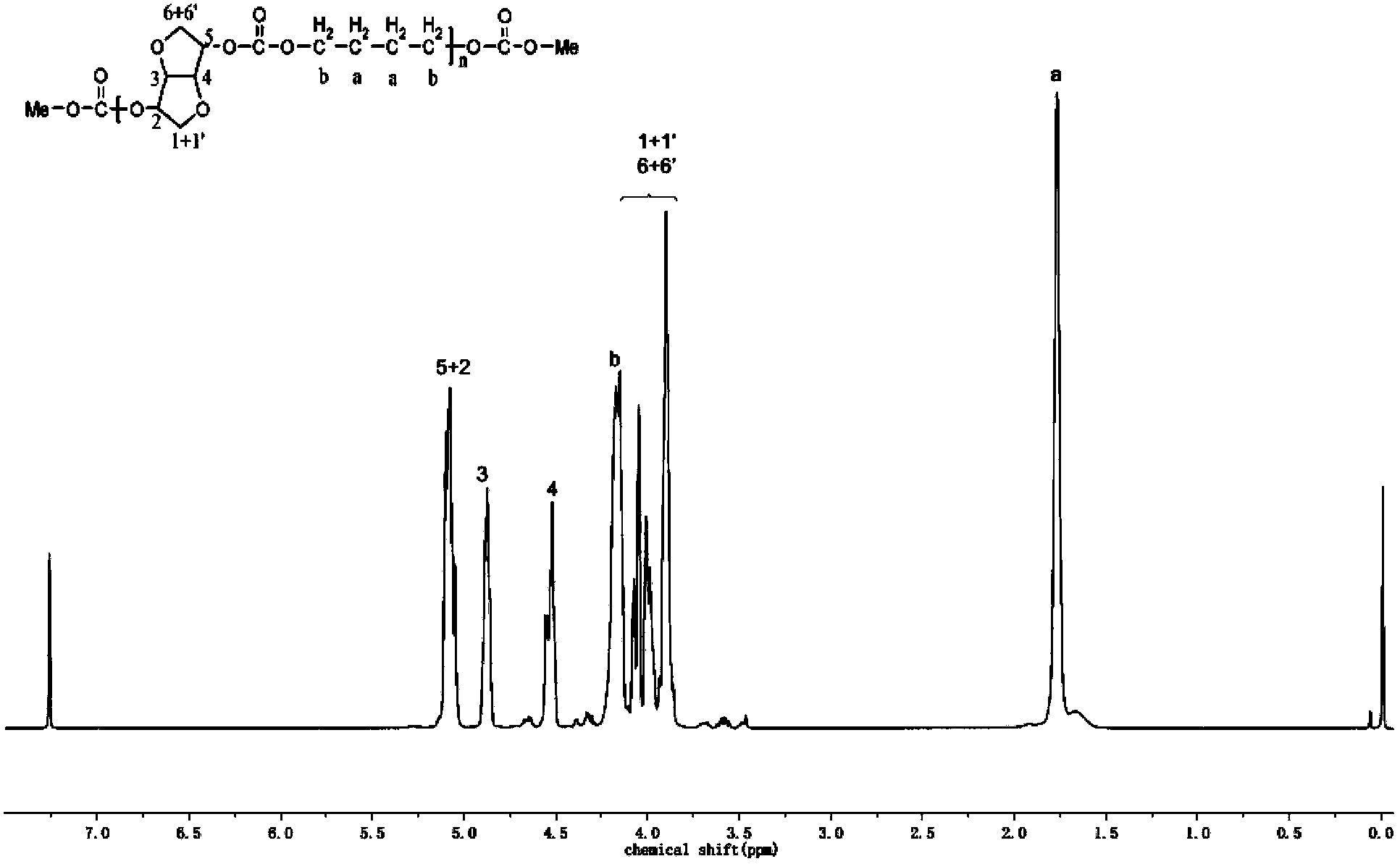
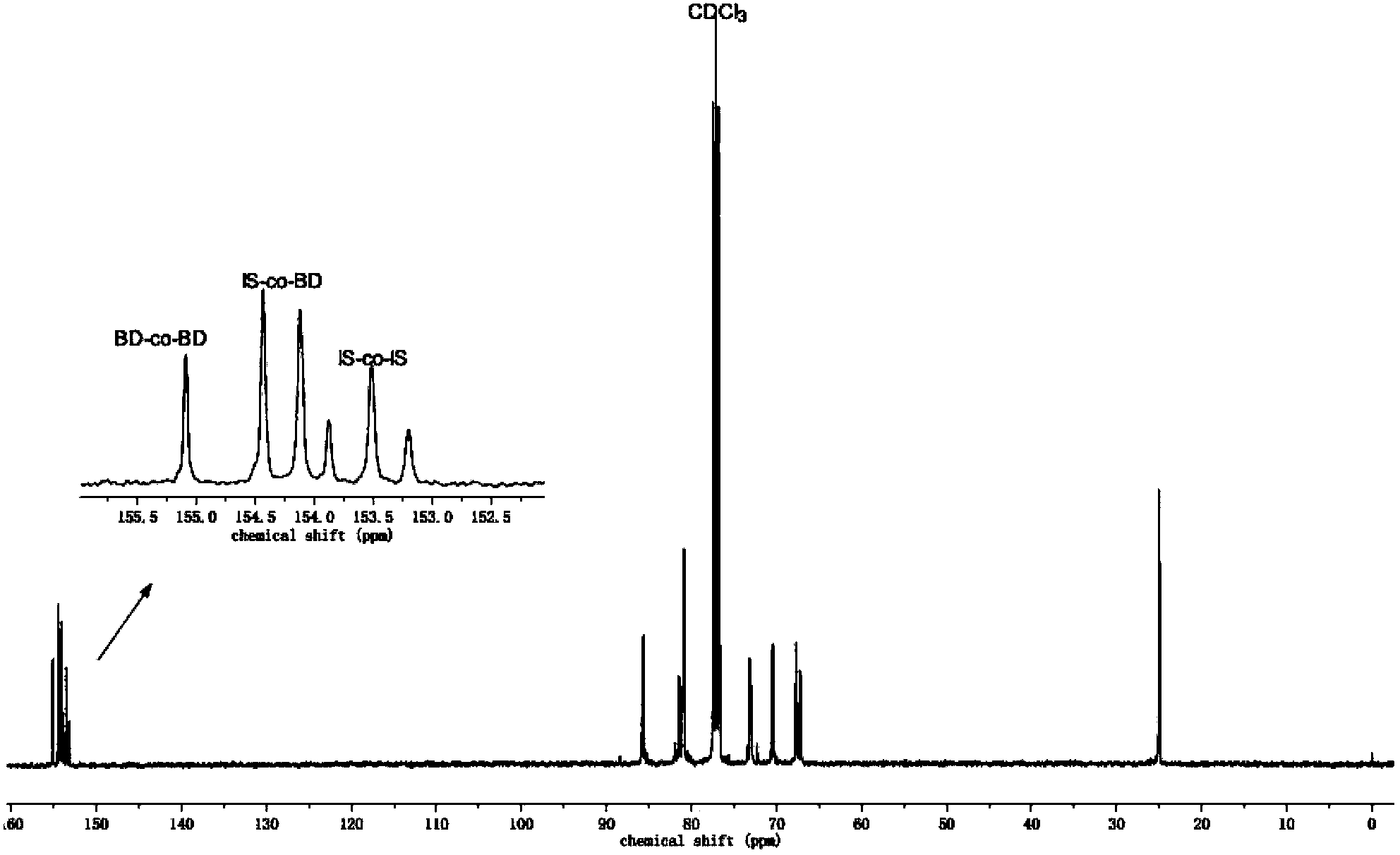
[0066] In a 250ml glass flask, under nitrogen atmosphere, add 180g dimethyl carbonate, 44g (0.3mol) isosorbide shown in formula II, 9g (0.1mol) aliphatic diol 1,4-butane shown in formula V Diol and 0.054g transesterification catalyst dibutyltin oxide (the molar ratio of dimethyl carbonate to diol is 5:1), stirring at 120°C for 4 hours, gradually raising the temperature to 220°C until the reaction is complete, no longer Methanol is distilled out to obtain the copolycarbonate prepolymer, then add 0.030g polycondensation catalyst sodium carbonate, polycondensate for 10h at 240°C and the pressure is lower than 200Pa, and finally obtain 45g copolycarbonate shown in formula I, its characteristics The viscosity number is 0.44dl / g, and the number average molecular weight is 28000. The H NMR spectrum of the polymer is figure 1 shown. The carbon nuclear magnetic spectrum proves that the copolycarbonate is distributed in ...
Embodiment 2
[0068] Embodiment 2, copolymer shown in preparation formula I
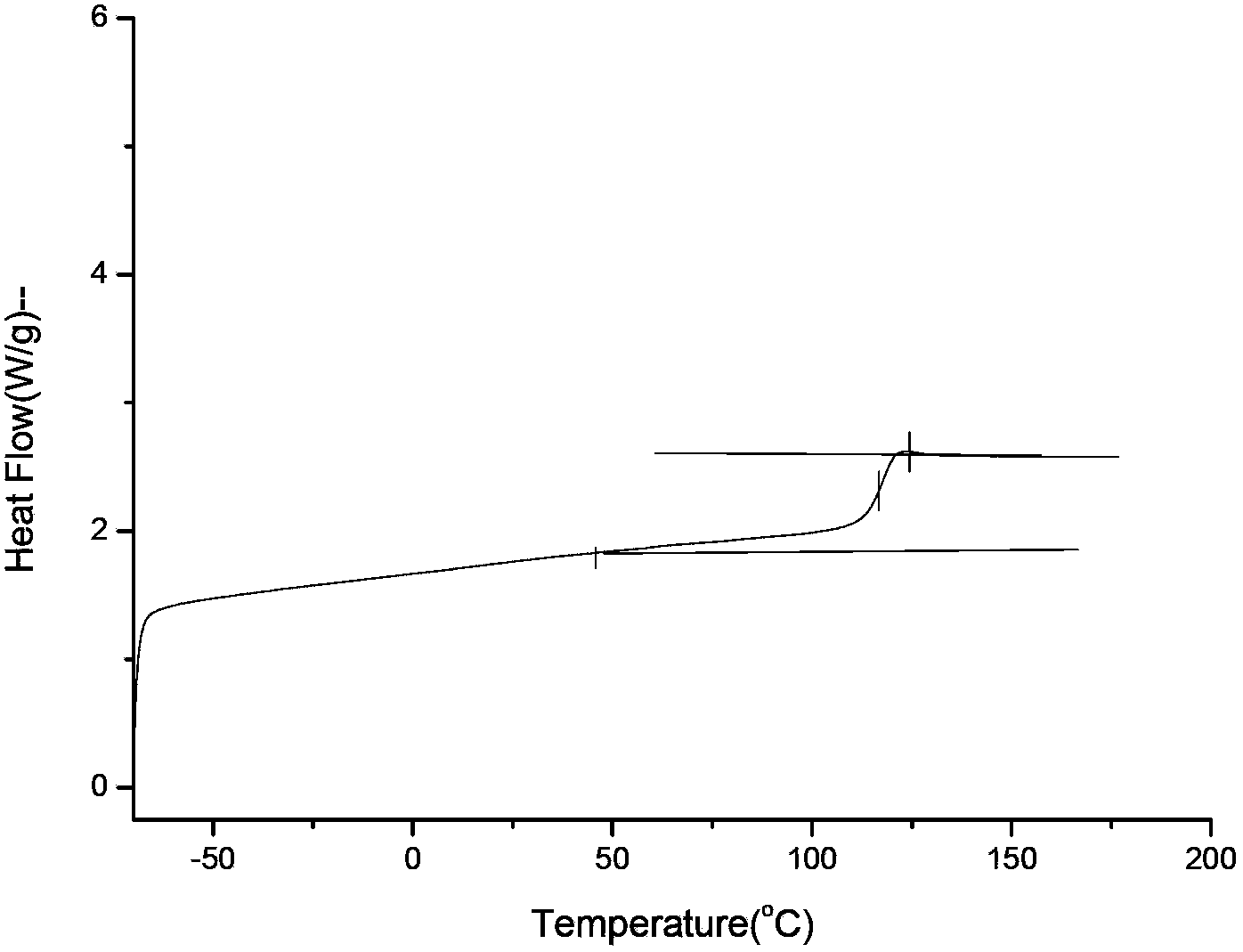
[0069] In a 250ml glass flask, under a nitrogen atmosphere, add 65g dimethyl carbonate, 47g (0.32mol) isosorbide shown in formula II, 4.5g (0.04mol) aliphatic diol 1,5- Pentylene glycol and 0.002g transesterification catalyst potassium carbonate (the molar ratio of dimethyl carbonate to diol is 2:1) (the molar ratio of dimethyl carbonate to diol is 5:1), stirring at 120°C to start the reaction 6h, gradually increase the temperature to 220°C until the reaction is complete, no more methanol will be distilled out to obtain a copolycarbonate prepolymer; add 0.030 polycondensation catalyst sodium carbonate, polycondense at 240°C for 15h under the condition of a pressure lower than 200Pa, and finally obtain 45g of copolycarbonate represented by formula I has an intrinsic viscosity of 0.43dl / g, a number average molecular weight of 27000, and Tg=106°C.
[0070] Among them, 1,4:3,6-dihexanol is isosorbide, formula Ⅴ is 1,...
Embodiment 3
[0071] Embodiment 3, copolymer shown in preparation formula I
[0072] In a 250ml glass flask, under nitrogen atmosphere, add 135g dimethyl carbonate, 22g (0.15mol) isosorbide shown in formula II, 22g (0.15mol) isoidide shown in formula IV and 0.022g transesterification catalyst Potassium carbonate (the molar ratio of dimethyl carbonate to diol is 5:1), start the reaction with stirring at 120°C for 7h, gradually increase the temperature to 220°C, until the reaction is complete, no methanol will distill out, and the copolycarbonate pre- Polymer;
[0073] In a 250ml glass flask, under a nitrogen atmosphere, add 90g dimethyl carbonate, 9g (0.1mol) 1,4-butanediol and 0.009g tetrabutyl titanate (the molar ratio of dimethyl carbonate to butanediol 10:1), stirred at 85°C to start the reaction for 4 hours, and gradually raised the temperature to 210°C until the reaction was complete, no methanol was distilled out, and poly-1,4-butanediol carbonate prepolymer was obtained;
[0074] M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com