Drug composition resisting influenza virus A or enterovirus
A technology of influenza virus and enterovirus, applied in antiviral agents, medical raw materials derived from fungi, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
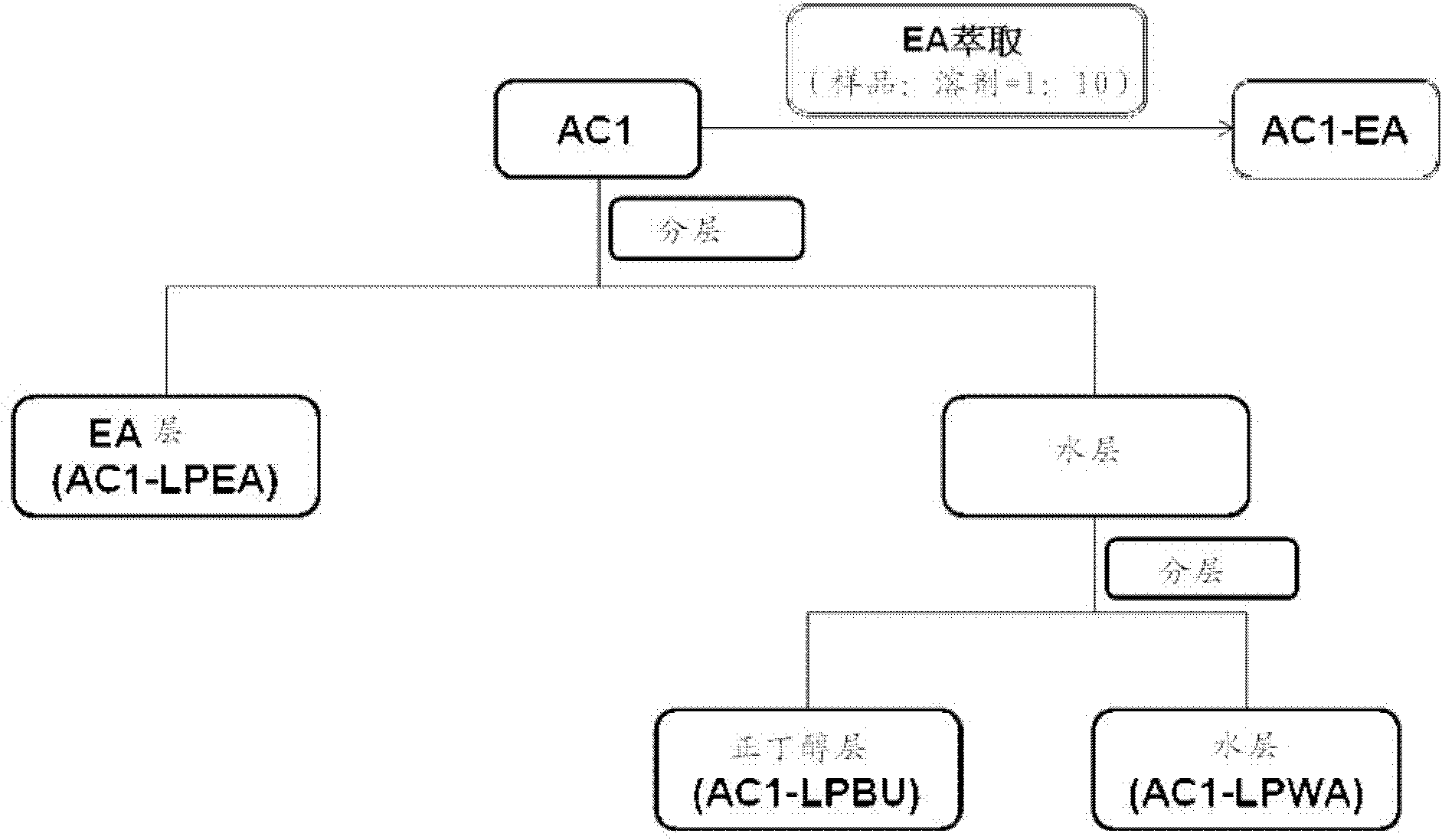
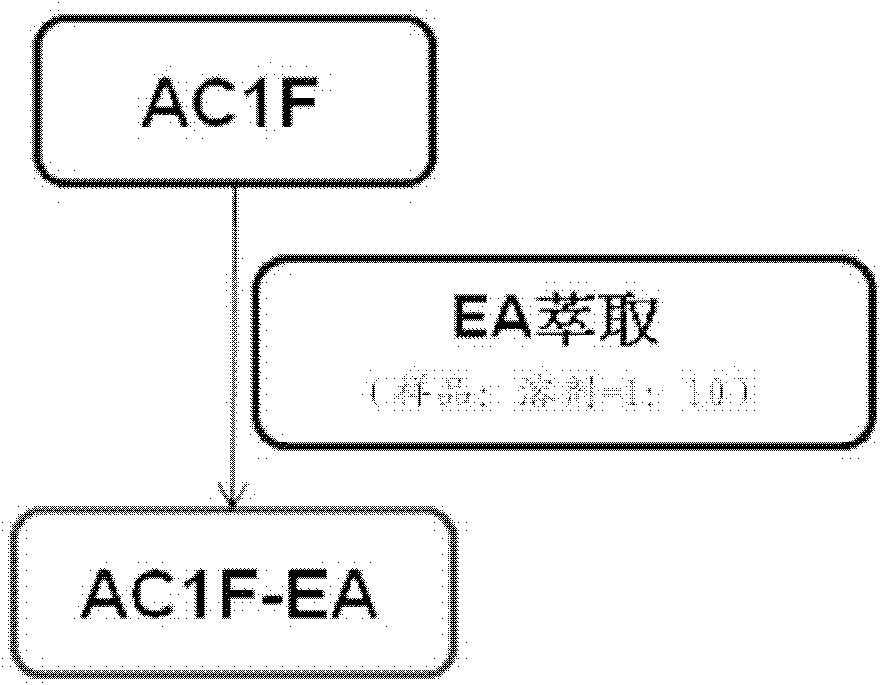
[0046] Fermentation broth AC1 and its mycelium AC1F:
[0047] 1. AC1 sub-extraction steps:
[0048] a: Put AC1 into a beaker, add 20 times the volume of M.Q water to redissolve, and pour it into a separatory funnel;
[0049] b: Add an equal volume of ethyl acetate (EA, which has been saturated with water) for partition. After the layers are separated, take out the upper layer (ie, the EA layer), and add an equal volume of water-saturated layer to the lower layer (ie, the water layer) EA continued to be sub-extracted, and after a total of 5 extractions, the 5 EA layers were combined and concentrated to obtain the EA layer sub-extract (AC1-LPEA);
[0050] c: The water layer after the EA fractionation continues to be sub-extracted with an equal volume of n-butanol (n-BuOH, which has been saturated with water), the method is the same as b, but the final combined n-butanol layer needs to be separated with an equal volume of n-butanol M.Q. water back extraction once, so that its n...
Embodiment 2
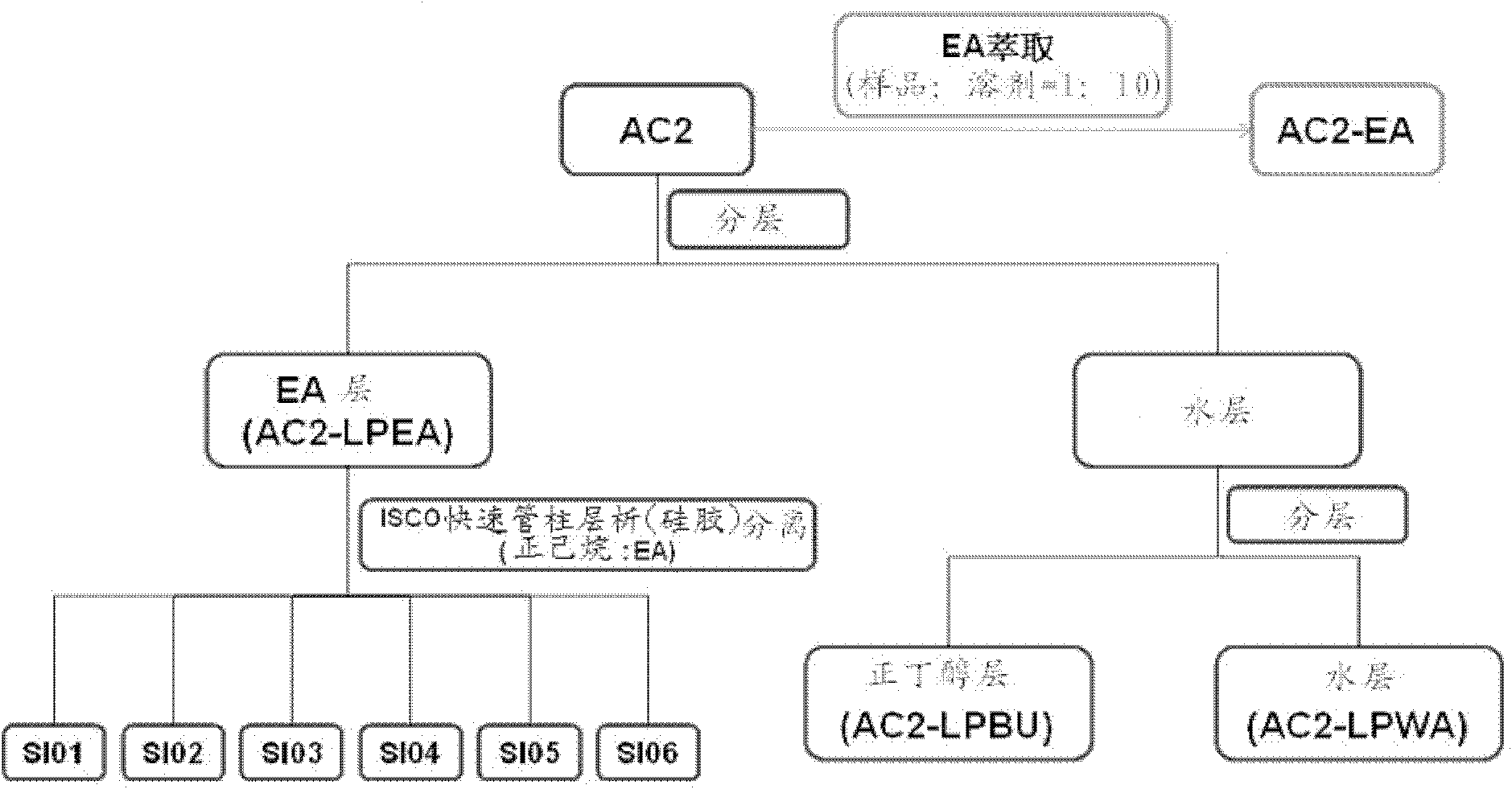
[0076] Fermentation broth AC2 and its mycelium AC2F
[0077] 1. AC2 sub-extraction steps:
[0078] a. Weigh AC2 in a beaker, add 20 times the volume of M.Q water to redissolve, and pour it into a separatory funnel.
[0079] b. Add an equal volume of EA (which has been saturated with water) for partitioning. After the layers are separated, take out the upper layer (ie, the EA layer), and add an equal volume of water-saturated EA to the lower layer (ie, the water layer) to continue the partition. After a total of 5 extractions, the 5 EA layers were combined and concentrated to obtain the EA layer sub-extract (AC2-LPEA).
[0080] c. The water layer after the EA fractionation continues to be sub-extracted with an equal volume of n-butanol (which has been saturated with water), the method is the same as b, but the final combined n-butanol layer needs to be back-extracted with an equal volume of M.Q. water Once, in this way, its n-butanol layer fraction (AC2-LPBU) can be obtained....
Embodiment 3
[0136] Fermentation broth AC3 and its mycelium AC3F:
[0137] AC3 fermentation broth and its mycelium extraction steps and process are the same as AC1.
[0138] 1. Antiviral activity test results: H1N1, H3N2 and EV71.
[0139] a.H1N1:
[0140]
[0141] b.H3N2:
[0142]
[0143] c. EV71:
[0144]
[0145] 2. Results:
[0146] a. AC3 has anti-H1N1 activity at a concentration of 200 μg / mL, but has no anti-H3N2 and EV71 activity.
[0147] b. The aqueous layer (AC1-LPWA) after AC3 fractionation has no activity; the EA layer (AC3-LPEA) has anti-H1N1 (50 μg / mL), H3N2 (50 μg / mL) and EV71 (100 μg / mL) activity; positive The butanol layer (AC3-LPBU) has anti-H1N1 (25μg / mL) and anti-EV71 (100μg / mL) activities, but has no activity against H3N2.
[0148] c. Directly extracted with EA, the fermentation broth AC3-EA has strong anti-H1N1 (100μg / mL) and EV71 (6.25μg / mL) activity, and has a slight anti-H3N2 (200μg / mL) activity, while mycelia AC3F-EA has anti-H1N1 (25 μg / mL) and E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com