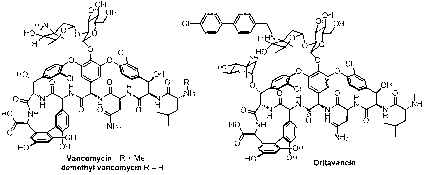
Vancomycin or demethyl vancomycin analogue and medicinal uses thereof
A technology of norvancomycin and vancomycin, applied in antibacterial drugs, drug combinations, anti-infective drugs, etc., can solve the problems of severe patients and increased mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
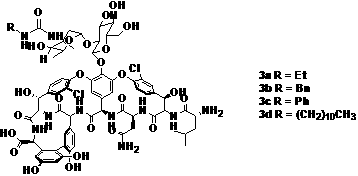
[0026] Example 1 Preparation of new vancomycin or norvancomycin analogs,
[0027] The compound is prepared according to the preparation methods known in the art 2a-d .
[0028] Add vancomycin hydrochloride (90mg, 0.061mmol) in 5ml reaction tube, mix solvent (Py:H 2 O=1:1) After 2ml is dissolved, add ethyl isocyanate (7.1 l, 0.091mmol), reacted at 30°C for 4h. The solvent was evaporated to dryness under reduced pressure, and the residue was purified by reverse-phase silica gel column chromatography with CH 3 OH:H2 O=1:1 elution gave carboyl vancomycin (2a) 62 mg with a yield of 53%.
[0029] According to the same method, the compounds represented by 2b-d were prepared in high yield.
[0030]
[0031] Table 1 Compounds 2a-d Mass spectral data (ESI-MS)
[0032] Numbering measured value Calculated value (M+Na + ) 2a 1541.5 1541.5 (C 69 h 80 Cl 2 N 10 o 25 Na) 2b 1603.1 1603.5 (C 74 h 82 Cl 2 N 10 o 25 Na) 2c 1589...
Embodiment 2
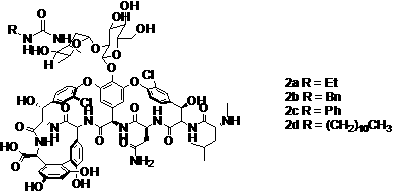
[0033] Example 2 Preparation of new vancomycin or norvancomycin analogs,
[0034] The compound is prepared according to the preparation methods known in the art 3a-d .
[0035] Take the compound N-Fmoc norvancomycin (100mg, 0.06mmol) and dissolve it in 2ml of mixed solvent (Py:DMF =1:1), add ethyl isocyanate (6.4ul, 0.09mmol) dropwise, react at room temperature for 5h, and reduce pressure Evaporate most of the solvent, add 20ml of diethyl ether to precipitate a white solid, filter off the diethyl ether, and wash the solid with 2*20ml of diethyl ether. The solid was dried to obtain 98.8 mg of the compound with a yield of 94.8%. Add the above solid (90mg, 0.061mmol) into a 5ml reaction tube, dissolve it with 1ml DMF, add dropwise 0.2ml of piperidine, and react at 10°C for 12h. The reaction solution was subjected to silica gel column chromatography, and CHCl 3 :CH 3 OH:H 2 O=4:4:0.7 eluted compound ( 3a) 72 mg, the yield was 91.8%.
[0036] According to the same method,...
Embodiment 3
[0040] Example 3 In vitro antibacterial activity test
[0041] The antibacterial activity of vancomycin analogues against Clostridium difficile IQCC23903, ATCC700057 and ATCC43255 was tested in vitro. Methods The minimum inhibitory concentration of antibacterial drugs (Minimal Inhibitory concentration MIC) was determined according to the agar double dilution method recommended by CLSI (Clinical Laboratory Standards Institute) in 2006. Experimental design: Take 1ml of antibacterial drugs of different types and different concentrations and pour them into a 9cm sterile empty plate, then pour 19ml of sterile M-H agar cooled to about 55°C on the plate immediately, mix well with the drug solution, and make the culture The final concentrations of base antibacterial drugs were 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125, 0.06 μg / ml; at the same time, M-H plates without antibacterial drugs were prepared as a control . Bacterial inoculation Add the bacteria that have been incubated ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap