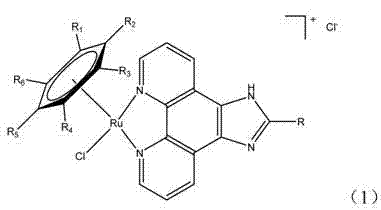
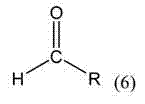
Microwave-assisted synthesis method for ruthenium (II) arene compound
A microwave-assisted synthesis of aromatic hydrocarbons, applied in the field of microwave-assisted chemical synthesis, can solve the problem of low reaction yield, achieve the effects of shortened reaction time, increased reaction yield, and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The structural formula of the phenanthrimidazole derivative PIP is as follows:
[0050]
[0051] The microwave-assisted synthesis method is: o-phenanthroline-5,6-dione (315mg, 1.50mmol), benzaldehyde (238.5mg, 2.25mmol), 4.5g ammonium acetate, 20mL glacial acetic acid in a microwave reaction tube, 100 Microwave radiation at ℃ for 20min; after the reaction, the reaction solution was poured into 27mL of distilled water, the pH value was adjusted to 7 with concentrated ammonia while stirring, a large amount of precipitate was obtained, filtered and dried to obtain a yellow crude product with a yield of 93.8% and a melting point of 320 ~323℃; ESI-MS (in EtOH, m / z): experimental value: 297.4 (theoretical value: 297.1); IR (KBr tablet) ν: 3064, 2965, 2839, 1602, 1563, 1477, 1459, 1350 , 1046, 951, 875, 808, 739, 704 cm -1 ; UV-vis(EtOH): 225nm, 274nm, 289nm.
Embodiment 2
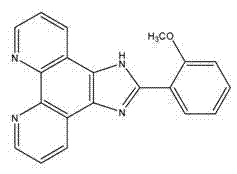
[0053] Phenanthrimidazole derivatives o -MOPIP, its structural formula is as follows:
[0054]
[0055] Phenanthroline-5,6-dione (315mg, 1.50mmol), 2-methoxybenzaldehyde (306mg, 2.25mmol), 4.5g ammonium acetate, 20mL glacial acetic acid in a microwave reaction tube, microwave at 100°C Irradiate for 20 min; after the reaction, pour the reaction solution into 27 mL of distilled water, adjust the pH value to 7 with concentrated ammonia while stirring, obtain a large amount of precipitate, filter, and dry to obtain a yellow crude product with a yield of 92.7%. Melting point: 270~274℃; ESI-MS (in EtOH, m / z): experimental value: 327.4 (theoretical value: 327.1); IR (KBr tablet) v: 3081, 2977, 1603, 1554, 1484, 1458, 1382 , 1043, 871, 815, 733, 698 cm -1 ; UV(EtOH): 225nm, 276nm, 290nm.
Embodiment 3
[0057] Phenanthrimidazole derivatives m -MOPIP, its structural formula is as follows:
[0058]
[0059] Phenanthroline-5,6-dione (315mg, 1.50mmol), 3-methoxybenzaldehyde (306mg, 2.25mmol), 4.5g ammonium acetate, 20mL glacial acetic acid in a microwave reaction tube, microwave at 100°C Irradiate for 20 minutes; after the reaction, pour the reaction solution into 27 mL of distilled water, adjust the pH to 7 with concentrated ammonia under stirring, obtain a large amount of precipitate, filter, and dry to obtain a yellow crude product with a yield of 91.3%. Melting point: 269~273℃; ESI-MS (in EtOH, m / z): experimental value: 327.4 (theoretical value: 327.1); IR (KBr tablet) v: 3087, 2972, 1598, 1561, 1481, 1456, 1376, 1032, 863, 805, 726, 714 cm -1 ; UV(EtOH): 225nm, 274nm, 289nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com