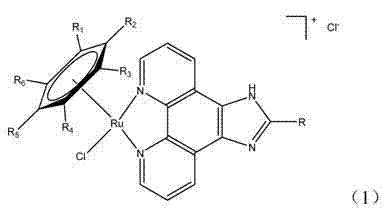
Microwave-assisted synthesis method for ruthenium (II) arene compound
A technology for synthesizing aromatic hydrocarbons and microwave assisted, applied in the field of microwave assisted chemical synthesis, can solve problems such as low reaction yield, and achieve the effects of shortening reaction time, improving reaction yield and huge application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
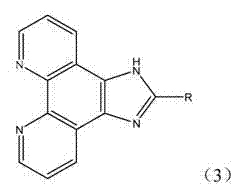
[0049] PIP, a phenanthroimidazole derivative, has the following structural formula:
[0050]
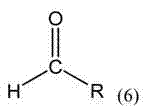
[0051] Its microwave-assisted synthesis method is: o-phenanthroline-5,6-dione (315mg, 1.50mmol), benzaldehyde (238.5mg, 2.25mmol), ammonium acetate 4.5g, glacial acetic acid 20mL in a microwave reaction tube, 100 Microwave radiation at ℃ for 20 minutes; after the reaction, pour the reaction solution into 27 mL of distilled water, adjust the pH value to 7 with concentrated ammonia water under stirring, a large amount of precipitation was obtained, filtered, and dried to obtain a yellow crude product with a yield of 93.8% and a melting point of 320 ~323℃; ESI-MS(in EtOH, m / z): Experimental value: 297.4 (theoretical value: 297.1); IR (KBr pellet) ν: 3064, 2965, 2839, 1602, 1563, 1477, 1459, 1350 , 1046, 951, 875, 808, 739, 704 cm -1 ; UV-vis (EtOH): 225nm, 274nm, 289nm.
Embodiment 2
[0053] Phenanthroimidazole Derivatives o -MOPIP, its structural formula is as follows:
[0054]
[0055] O-phenanthroline-5,6-dione (315mg, 1.50mmol), 2-methoxybenzaldehyde (306mg, 2.25mmol), ammonium acetate 4.5g, glacial acetic acid 20mL in a microwave reaction tube, microwave at 100°C Irradiate for 20 minutes; after the reaction, pour the reaction solution into 27 mL of distilled water, adjust the pH value to 7 with concentrated ammonia water under stirring, a large amount of precipitation is obtained, filter, and dry to obtain a yellow crude product with a yield of 92.7%. Melting point 270~274℃; ESI-MS(in EtOH, m / z): Experimental value: 327.4 (theoretical value: 327.1); IR (KBr pellet) v: 3081, 2977, 1603, 1554, 1484, 1458, 1382 , 1043, 871, 815, 733, 698 cm -1 ; UV(EtOH): 225nm, 276nm, 290nm.
Embodiment 3
[0057] Phenanthroimidazole Derivatives m -MOPIP, its structural formula is as follows:
[0058]
[0059] O-phenanthroline-5,6-dione (315mg, 1.50mmol), 3-methoxybenzaldehyde (306mg, 2.25mmol), ammonium acetate 4.5g, glacial acetic acid 20mL in a microwave reaction tube, microwave at 100°C Irradiate for 20 minutes; After the reaction, pour the reaction solution into 27 mL of distilled water, adjust the pH value to 7 with concentrated ammonia water under stirring, a large amount of precipitation is obtained, filter, and dry to obtain a yellow crude product with a yield of 91.3%. Melting point 269~273°C; ESI-MS (in EtOH, m / z): experimental value: 327.4 (theoretical value: 327.1); IR (KBr pellet) v: 3087, 2972, 1598, 1561, 1481, 1456, 1376, 1032, 863, 805, 726, 714 cm -1 ; UV(EtOH): 225nm, 274nm, 289nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com