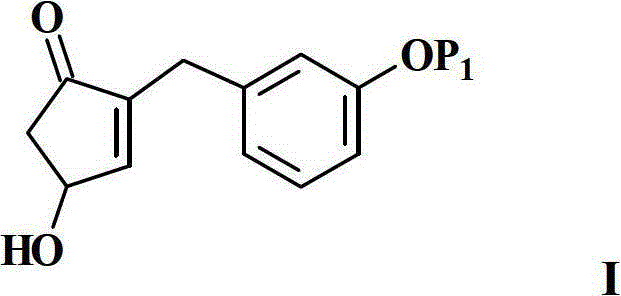
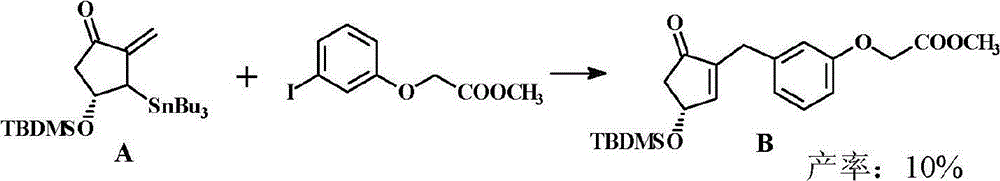Preparation method of cyclopentenone and cyclopentenone for synthesizing benzindene prostaglandins
A technology of cyclopentenone and phenyl, which is applied in the direction of preparation of heterocyclic compounds, chemical instruments and methods, compounds of group 4/14 elements of the periodic table, etc., can solve problems such as expensive, low yield, and not easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0060] 1-(furan-2-yl)-2-(3-methoxyphenyl)ethan-1-one
[0061]
[0062] 3-Methoxyphenylacetic acid (20 g, 120.35 mmol) was dissolved in CH 2 Cl 2 (200 mL), and to the solution were added furan (24.6 g, 361.39 mmol) and trifluoroacetic anhydride (37.8 g, 180 mmol). The reaction was kept stirring overnight at room temperature. with saturated NaHCO 3 The reaction was quenched until pH = 6.9, and the organic reagents in the mixture were evaporated. The residue was extracted with EtOAc, and the organic layer was washed with brine, washed over MgSO 4 Dry and evaporate. The resulting residue was purified by silica gel column chromatography using hexane-EtOAc as eluent to afford 1-(furan-2-yl)-2-(3-methoxyphenyl)ethane-1- as a yellow oil Ketone: 20g (yield: 77%)
[0063] 1 HNMR (300MHz, CDCl 3)δ3.76(3H,s),4.07(2H,s),6.50(1H,dd,J=1.7,3.6Hz),6.76-6.89(3H,m),7.19-7.26(2H,m),7.57 (1H,dd,J=0.7,1.7Hz).
example 2
[0065] 1-(furan-2-yl)-2-(3-benzyloxyphenyl)ethan-1-one
[0066]
[0067] 3-Benzyloxyphenylacetic acid (3.5kg, 14.45mol) was dissolved in CH 2 Cl 2 (35 L), and furan (3.0 kg, 44 mol) and trifluoroacetic anhydride (4.55 kg, 21.65 mol) were added to the solution. The reaction was kept stirring at room temperature for 2 days. with saturated NaHCO 3 The reaction was quenched until pH = 6.9, and the organic reagents in the mixture were evaporated. The residue was extracted with EtOAc, and the organic layer was washed with brine, washed over MgSO 4 Dry and evaporate. The resulting residue was purified by silica gel column chromatography using hexane-EtOAc as eluent to afford 1-(furan-2-yl)-2-(3-benzyloxyphenyl)ethane-1 as a yellow oil - Ketone: 4kg (yield: 95%)
[0068] 1 HNMR (300MHz, CDCl 3 )δ4.08(2H,s),5.03(2H,s),6.50(1H,dd,J=1.71,3.59Hz),6.86-6.97(3H,m),7.19-7.44(7H,m),7.51 (1H,dd,J=0.76,1.7Hz)
[0069] 13 CNMR (75MHz, CDCl 3 )δ45.3, 69.8, 112.3, 113.3, 115.9, 118...
example 3
[0071] 1-(furan-2-yl)-2-(3-methoxyphenyl)ethan-1-ol
[0072]
[0073] 1-(furan-2-yl)-2-(3-methoxyphenyl)ethan-1-one (20 g, 92.56 mmol) was dissolved in MeOH (200 mL) and slowly added to the solution at 5 °C Add NaBH 4 (3.5 g, 92.52 mmol). After the reaction is complete, use H 2 The solution was quenched with O, and MeOH was evaporated. The residue was extracted with EtOAc and the organic layer was washed with brine, washed over MgSO 4 Drying and evaporation gave 1-(furan-2-yl)-2-(3-methoxyphenyl)ethan-1-ol as a yellow oil: 20 g (crude yield: 98%)
[0074] 1 HNMR (300MHz, CDCl 3 )δ2.12(1H,s),3.15(2H,m),3.79(3H,s),4.93(1H,s),6.25(1H,s),6.35(1H,s),6.74(1H,s ),6.81(2H,m),7.23(1H,t,J=7.9Hz),7.43(1H,s)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com