Domperidone maleate tablet and preparation process thereof
A technology of perperidone tablet and maleic acid, applied in the field of domperidone maleate tablet and preparation thereof, can solve the problems of inconvenient taking of ordinary tablets, drug precipitation, increase production cost and the like, achieve the effect of improving dissolution, increase Stability and superior quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
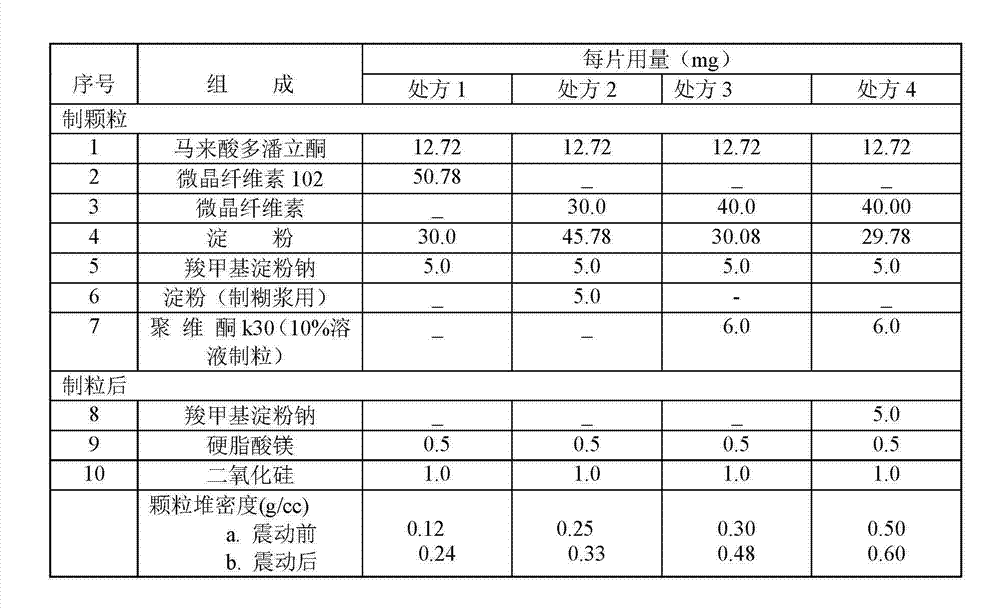
[0017]
[0018] Preparation Process
[0019] Pass domperidone maleate and all auxiliary materials through a 100-mesh sieve, and set aside; weigh the prescribed amount of microcrystalline cellulose, starch, sodium carboxymethyl starch, pass through a 80-mesh sieve and mix with the main ingredient, and add povidone K30 The pure aqueous solution made of 38ml of pure water is a binder soft material, granulated with a 16-mesh sieve, dried at 65°C for 1 hour, and granulated at 16 mesh; Slice and serve.
reference example 2
[0021]
[0022] Preparation Process
[0023] Pass domperidone maleate and all auxiliary materials through a 100-mesh sieve respectively, and set aside; weigh the prescribed amount of microcrystalline cellulose, starch, and half of the sodium carboxymethyl starch, pass through a 80-mesh sieve and mix with the main ingredient, and use polyvinyl chloride The pure aqueous solution prepared by adding 38ml of pure water to ketone K30 is a soft material made of adhesive, granulated with a 16-mesh sieve, dried at 65°C for 1 hour, and granulated with a 16-mesh; weigh the prescribed amount of silicon dioxide, magnesium stearate and Half of the prescription amount of sodium carboxymethyl starch is blended and compressed into tablets.
Embodiment 1
[0025]
[0026] Preparation Process
[0027] Pass domperidone maleate and all auxiliary materials through a 100-mesh sieve respectively, and set aside; weigh the prescribed amount of microcrystalline cellulose, starch, and half of the sodium carboxymethyl starch, pass through a 80-mesh sieve and mix with the main ingredient, and use polyvinyl chloride The pure aqueous solution prepared by adding 38ml of pure water to ketone K30 is a soft material made of adhesive, granulated with a 16-mesh sieve, dried at 65°C for 1 hour, and granulated with a 16-mesh; weigh the prescribed amount of silicon dioxide, magnesium stearate and Half of the prescription amount of sodium carboxymethyl starch is blended and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com