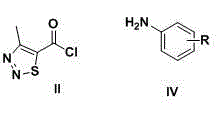
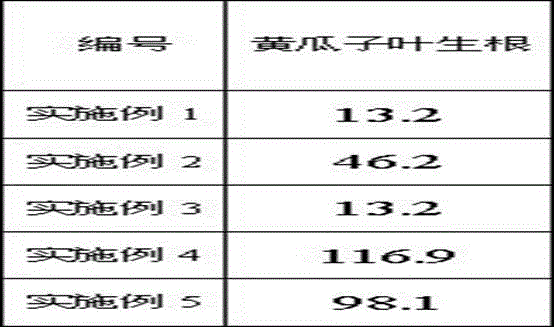
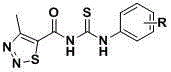
Thiadiazole thiourea derivative as well as preparation and application thereof as plant growth regulator
A technology of thiadiazole and derivatives is applied to thiadiazole thiourea derivatives and their preparation and application fields as plant growth regulators, and can solve problems such as strong toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0040] Example 1: Preparation of N-(5-(2,5-dichlorophenyl)-N'-(4-methyl-1,2,3-thiadiazol-5-yl)-thiourea
[0041] In a 100mL round bottom flask, add 0.97g (10mmol) of NH 4 NCS dichloromethane PEG-600 solution (10mL, including 9.5 mL dichloromethane and 0.5 mL PEG-600), stirred and refluxed for 15 minutes, left to cool, and suction filtered to obtain an orange solution, and added 10 mmol of the above method 2,5-Dichloroaniline was dissolved in 5 mL of anhydrous acetonitrile, stirred at room temperature for 10 h, allowed to stand overnight, filtered with suction, and the filter cake was recrystallized with a mixed solvent of DMF and water at a volume ratio of 1:1 to obtain the product.
[0042] Yellow solid, yield 85.9 %, melting point%: 181-183 ℃; 1 H NMR: 8.35 (s, 1H, NH), 8.14 (s, 1H, NH), 7.39-7.44 (m, 1H, Ph), 7.18-7.30 (m, 1H, Ph), 7.13-7.17 (m, 1H , Ph), 2.81 (s, 3H, CH 3); ESI-MS: 346 (M-1) ; Anal. calcd. For C 11 h 8 Cl 2 N 4 OS 2 : C 38.05, H 2.32, N 16.13; fou...
example 2
[0043] Example 2: Preparation of N-(5-(4-fluorophenyl)-N'-(4-methyl-1,2,3-thiadiazol-5-yl)-thiourea
[0044] In a 100mL round bottom flask, add 0.97g (10mmol) of NH 4 NCS dichloromethane PEG-600 solution (10mL, of which dichloromethane 9.5 mL, PEG-600 0.5 mL), stirred and refluxed for 15min, left to cool, suction filtered to obtain an orange solution, added the prepared 10mmol 4- Dissolve fluoroaniline in 5 mL of anhydrous acetonitrile, stir at room temperature for 10 h, let stand overnight, filter with suction, take the filter cake and recrystallize it with a mixed solvent of DMF and water at a volume ratio of 1:2 to obtain the product.
[0045] Yellow solid, yield 83.8 %, melting point: 166-168 ℃; 1 H NMR: 11.96 (s, 1H, NH), 10.00 (s, 1H, NH), 7.63-7.66 (m, 1H, Ph), 7.26-7.29 (m, 2H, Ph), 2.80 (s, 3H, CH 3 ); ESI-MS: 295 (M-1) ; Anal. calcd. For C 11 h 9 FN 4 OS 2 : C 44.58, H 3.06, N 18.91; found: C 44.46, H 3.13, N 18.76.
example 3
[0046] Example 3: Preparation of N-(5-(3-nitrophenyl))-N'-(4-methyl-1,2,3-thiadiazol-5-yl)-thiourea
[0047] In a 100mL round bottom flask, add 0.97g (10mmol) of NH 4 NCS dichloromethane PEG-600 solution (10mL, of which dichloromethane 9.5 mL, PEG-600 0.5 mL), stirred and refluxed for 15min, left to cool, suction filtered to obtain an orange solution, added the prepared 10mmol 3- Nitroaniline was dissolved in 10 mL of anhydrous acetonitrile, stirred at room temperature for 10 h, allowed to stand overnight, filtered with suction, and the filter cake was recrystallized with a mixed solvent of DMF and water at a volume ratio of 2:1 to obtain the product.
[0048] Yellow solid, yield 85.8 %, melting point: 115-117 ℃; 1 H NMR: 12.20 (s, 1H, NH), 8.71 (s, 1H, NH), 8.13 (m, J =8.48Hz, 1H, Ph), 8.02 (d, J =7.72Hz, 1H, Ph), 7.71 (t, J =8.09Hz, 1H, Ph), 2.82 (s, 3H, CH 3 ); ESI-MS: 322 (M-1) ; Anal. calcd. For C 11 h 9 N 5 o 3 S 2 : C 40.86, H 2.81, N 21.66; found: C 40.89, H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com