Compound with 1,3,5-triazine ring structure and preparation method thereof
A compound, triazine ring technology, applied in the field of triazine compounds with 1, ultraviolet absorbers, can solve the problems of extremely high reaction requirements, harsh synthesis conditions, environmental hazards, etc., achieve mild reaction conditions, improve performance and use Longevity and anti-aging effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
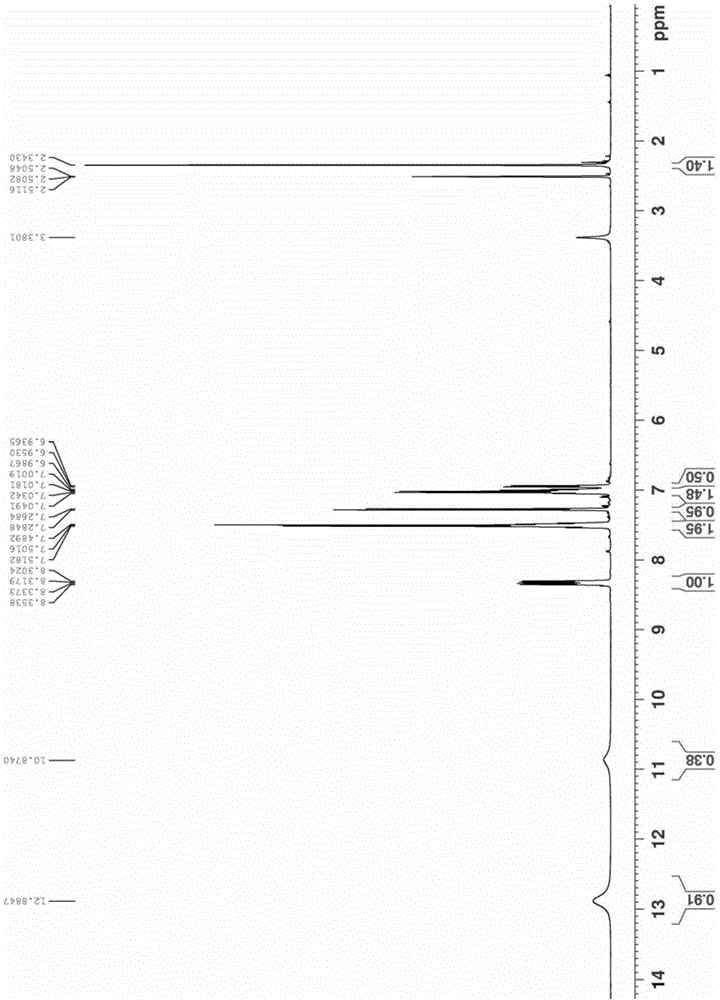
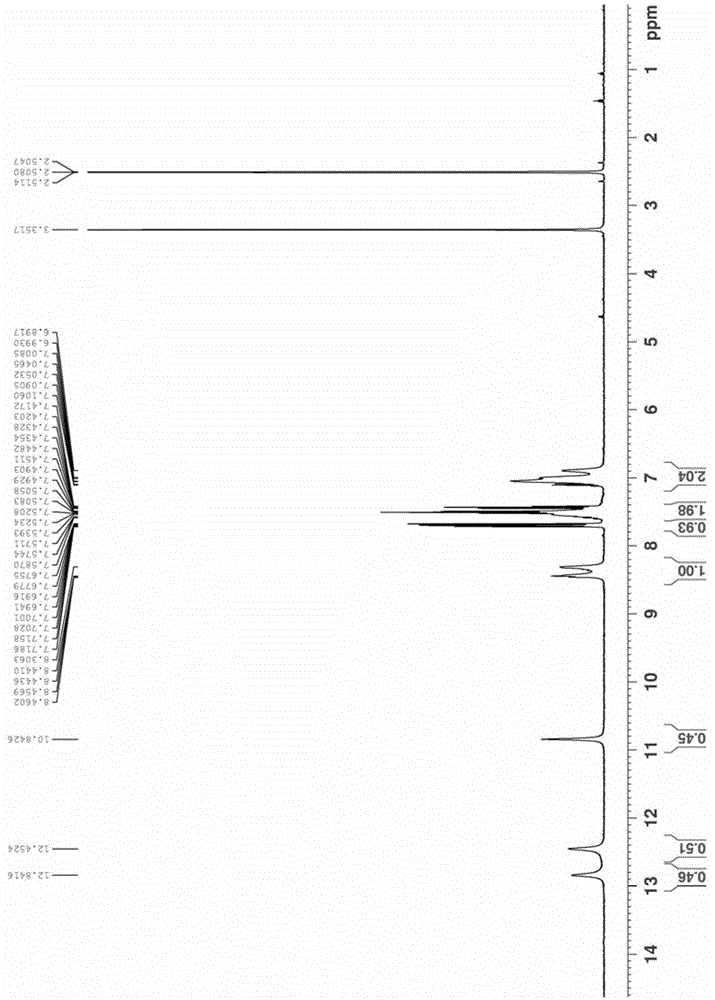
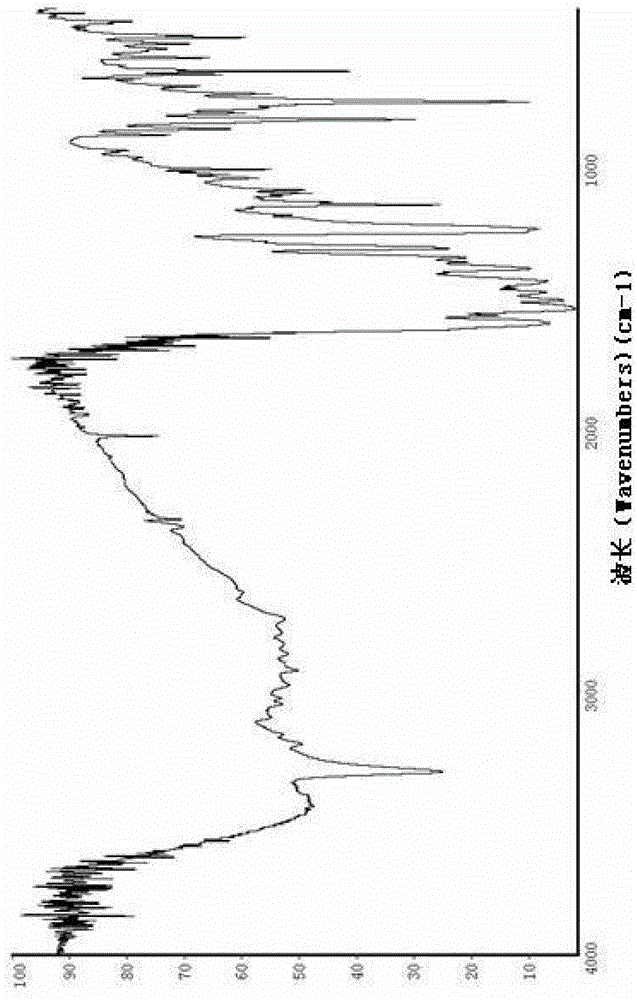
Image
Examples
Embodiment 1
[0045] Preparation of 2-(2-hydroxyphenyl)-[1,3]benzoxazinone
[0046]
[0047] Add 13.7g (0.1mol) salicylic acid amide and 21.4g (0.1mol) phenyl salicylate in the reaction bottle that distillation unit is housed, then heat up to 180 ℃ and make raw material react in molten state for 3 hours, the whole reaction The process is carried out under reduced pressure, and the water produced in the reaction process is removed by distillation under reduced pressure while reacting. After the reaction is over, 50mL of absolute ethanol solvent is added under melting to separate out a yellow solid product, which is filtered to obtain the corresponding product. To improve the purity of the product, add the obtained yellow solid product to 50mL of absolute ethanol solvent and heat up to dissolve, then stir for 15 minutes, then slowly cool down to 0°C-5°C for recrystallization for 30 minutes, crystallize, filter, Vacuum drying at 30°C yielded 16.8 g of the intermediate product 2-(2-hydroxyph...
Embodiment 2
[0050] Formula I compound 2,4-bis(2-hydroxyphenyl)-6-(4-methylanilino)-1,3,5-triazine of the following structural formula
[0051]
[0052] The preparation method of the formula I compound 2,4-bis(2-hydroxyphenyl)-6-(4-methylanilino)-1,3,5-triazine in this example is as follows:
[0053] Add 2-(2-hydroxyphenyl)-[1,3]benzoxazinone 2.39g (0.01mol) into the reaction flask equipped with a reflux device, and then add 4-methylbenzoguanidine hydrochloride 1.85 g (0.01mol), 0.5g (0.0125mol) of inorganic alkaline catalyst sodium hydroxide and 50mL of absolute ethanol solvent, heat up to reflux for 4 hours, after the reaction, cool down to 0℃~5℃, stir and crystallize After 30 minutes, a large amount of solids were precipitated, then filtered, washed with water, and the obtained wet solid product was put into an oven and dried under the condition of controlling the temperature at 80°C to obtain the corresponding product 2,4-bis(2-hydroxyphenyl) -6-(4-methylanilino)-1,3,5-triazine. I...
Embodiment 3
[0059] Formula I compound 2,4-bis(2-hydroxyphenyl)-6-(2-methoxyanilino)-1,3,5-triazine of the following structural formula
[0060]
[0061] The preparation method of the formula I compound 2,4-bis(2-hydroxyphenyl)-6-(2-methoxyanilino)-1,3,5-triazine in this example is as follows:
[0062] Add 2.39g (0.01mol) of 2-(2-hydroxyphenyl)-[1,3]benzoxazinone to the reaction flask equipped with a reflux device, and then add 2-methoxyphenylguanidine hydrochloride 2.01g (0.01mol), inorganic basic catalyst potassium hydroxide 0.73g (0.013mol) and 45mL of absolute ethanol solvent, heated to reflux for 8 hours, after the reaction, cooled to 0 ℃ ~ 5 ℃, stirred and analyzed After crystallization for 30 minutes, a large amount of solids were precipitated, then filtered, washed with water, and the obtained solid wet product was put into an oven and dried at a temperature of 80°C to obtain the corresponding product 2,4-bis(2-hydroxyphenyl )-6-(2-methoxyphenylamino)-1,3,5-triazine, in order t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com