Pharmaceutical composition for the preparation of an antibacterial preparation for infusion and a method for its preparation (variants)
A composition and drug technology, applied in the directions of drug combinations, antibacterial drugs, antifungal agents, etc., can solve the problems of not enhancing the therapeutic efficacy of antibacterial drugs, being less commonly used, and not having antibacterial effects, and achieving increased efficacy and quality, No waste cost, lower mortality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
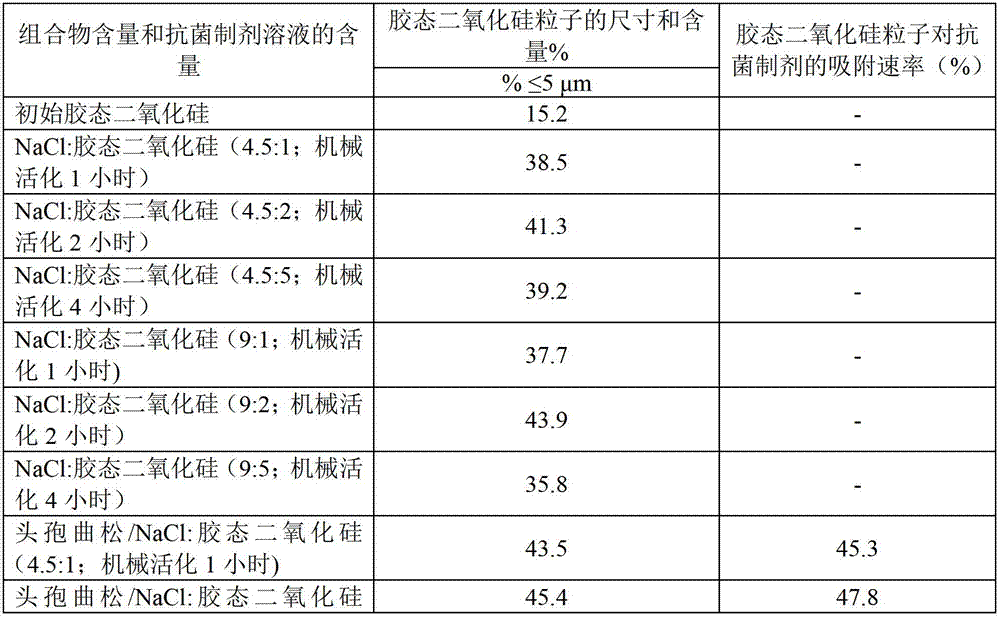
[0031] Example 1. Preparation of solid composition. NaCl: colloidal silicon dioxide.
[0032] Blends of sodium chloride and colloidal silica in weight ratios of 4.5:1, 4.5:2, 4.5:5, 9:1, 9:2, and 9:5 were processed in a rotary drum mill1 , 2 or 4 hours.
[0033] The particle content of suspensions of initial colloidal silica particles and their composition with NaCl in different variants in water was analyzed on a Microsizer-201а particle size laser analyzer manufactured by the Russian company «VA Instalt». Place 1g to 5g of the powder under study into the sample preparation module (liquid volume 150sm 3 ) in an amount sufficient to transmit 70% to 75% of the light through the test tube. After 1 or 2 minutes, the measurement is taken while the suspension is processed to break up clumps. Data processing is performed according to a calculation program embedded in the analyzer. The results are presented in the form of a histogram of weight distribution versus particle size. ...
example 2
[0040] Example 2. Preparation of solid composition
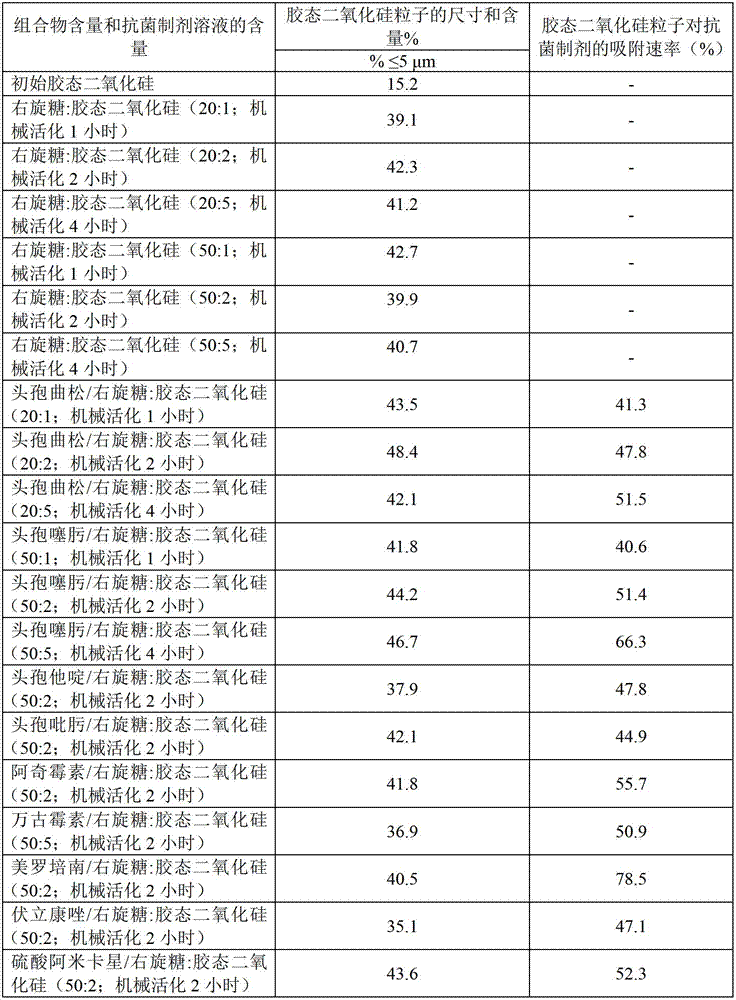
[0041] Dextrose: Colloidal silicon dioxide. Blends of dextrose and colloidal silica in weight ratios of 20:1, 20:2, 20:5, 50:1, 50:2, and 50:5 were processed in a rotary drum mill1 , 2 or 4 hours.
[0042] Following the method described in Example 1, the particle content of the suspension of colloidal silica in water and the adsorption rate of antibiotics were measured. The data obtained are shown in Table 2. From these data, it can be seen that the method for preparing the provided composition also increases the proportion of the colloidal silica fine powder fraction (particle size ≤ 5 μm) by at least 2 times and the binding of colloidal silica particles to the molecules of the antimicrobial preparation reached at least 40%.
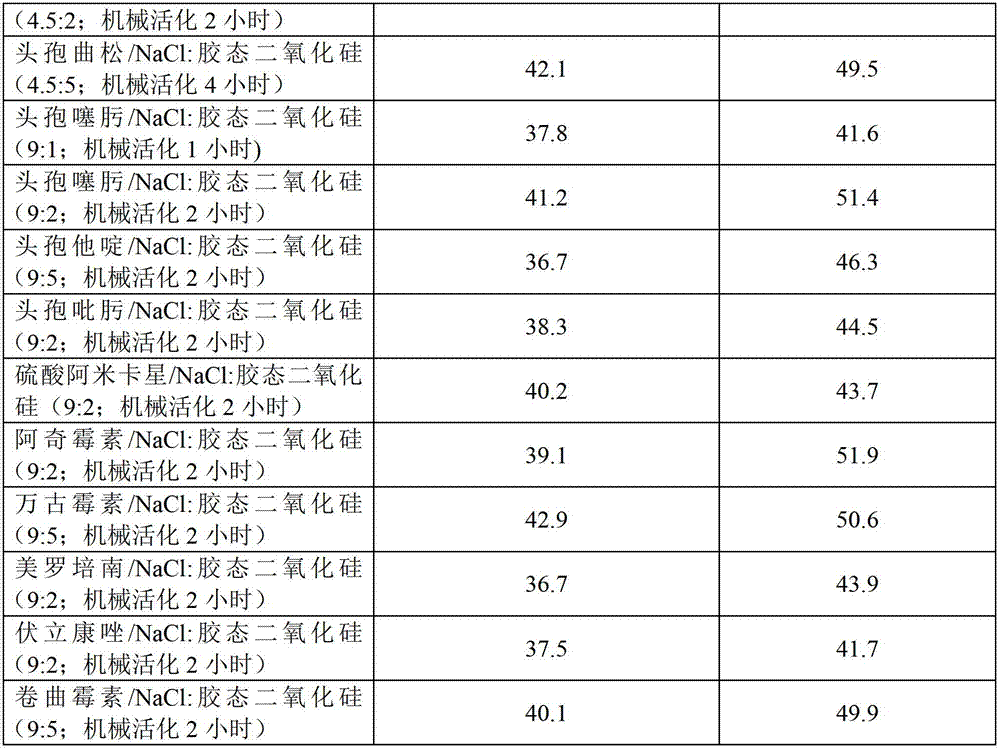
[0043] Table 2
[0044] Particle determination data of suspensions of compositions and solutions of antibacterial preparations in water prepared by application of the composition; adsorption rate...
example 3
[0046] Example 3. Determination of the therapeutic efficacy of a solution (for intravenous injection) based on an antibacterial preparation prepared using a pharmaceutical composition.
[0047] A study has been conducted against β-lactam antibiotics (amoxicillin + clavulanic acid, cefotaxime, ceftriaxone, cefoperazone + sulbactam, ceftazidime, cefepime, aztreonam, meropenem), Research on macrolides (azithromycin), aminoglycosides (amikacin sulfate), glycopeptides (vancomycin), antifungals (voriconazole), and fosfomycin.
[0048] In order to determine the therapeutic efficacy of antimicrobial agents, an experimental model of sepsis was first established, and according to [19, 20], the obtained results (χ 2 ) statistical processing method.
[0049] microorganism : Staphylococcus aureus (ATCC No. 25923F-49), Escherichia coli (ATCC No. 25922F-50), Pseudomonas aeruginosa (ATCC No. 27853F-51), Candida albicans (ATCC No. 24433).
[0050] animal : The experiment was based on "Reg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com