Crystal form, preparation method and application of prostaglandin analogue
一种晶型、药物的技术,应用在化学制药领域,能够解决前列腺素类化合物稳定性差等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The preparation method of compound I crystal form B
[0055] The present invention also provides a preparation method of the crystal form B of the compound represented by formula I.
[0056] In one embodiment provided by the present invention, the preparation method of the crystal form B of the compound of formula I comprises the following steps:
[0057] (1) mixing the crude product of the compound shown in formula I with solvent 1 to obtain solution 1; said solvent 1 is selected from the group consisting of acetone, isopropanol, n-propanol, tetrahydrofuran, or a mixture thereof,
[0058] (2) Cool down and stir the solution 1 to precipitate the crystal form B of the compound of formula I.
[0059] In step (1), the mixing should be performed below the boiling point of solvent 1, preferably at 20-60°C, more preferably at 40-50°C.
[0060] In step (1), the mixing ratio (weight to volume ratio) of the crude product of the compound represented by formula I and solvent 1 i...
Embodiment 1
[0099] Preparation of crude compound I
[0100] With reference to the preparation method reported in the document J.Org.Chern.2004, 69, 1890-1902, (1R, 2R, 3aS, 9aS)-2,3,3a,4,9,9a-hexahydro-1-[( 3S)-3-Hydroxyoctyl]-1H-phenyl[f]indene-2,5-diol was used as the starting material, and 41 g of crude compound I was obtained without purification.
Embodiment 4
[0106] Preparation of compound I crystal form B
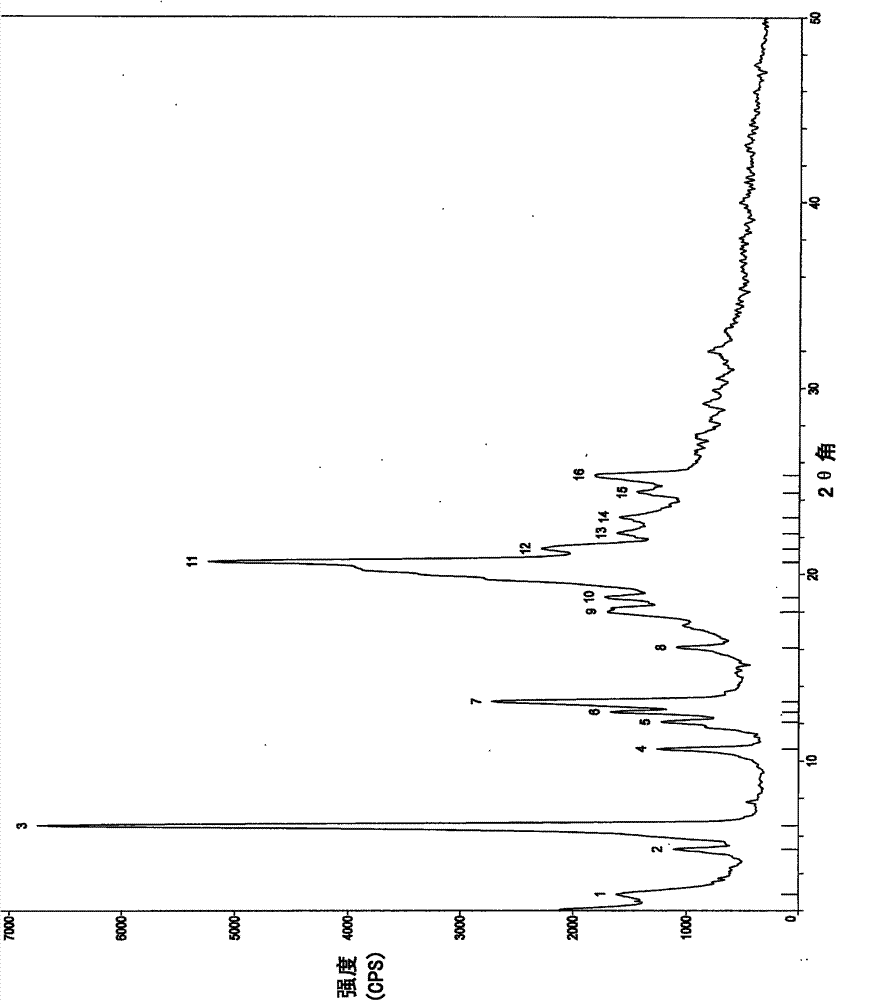
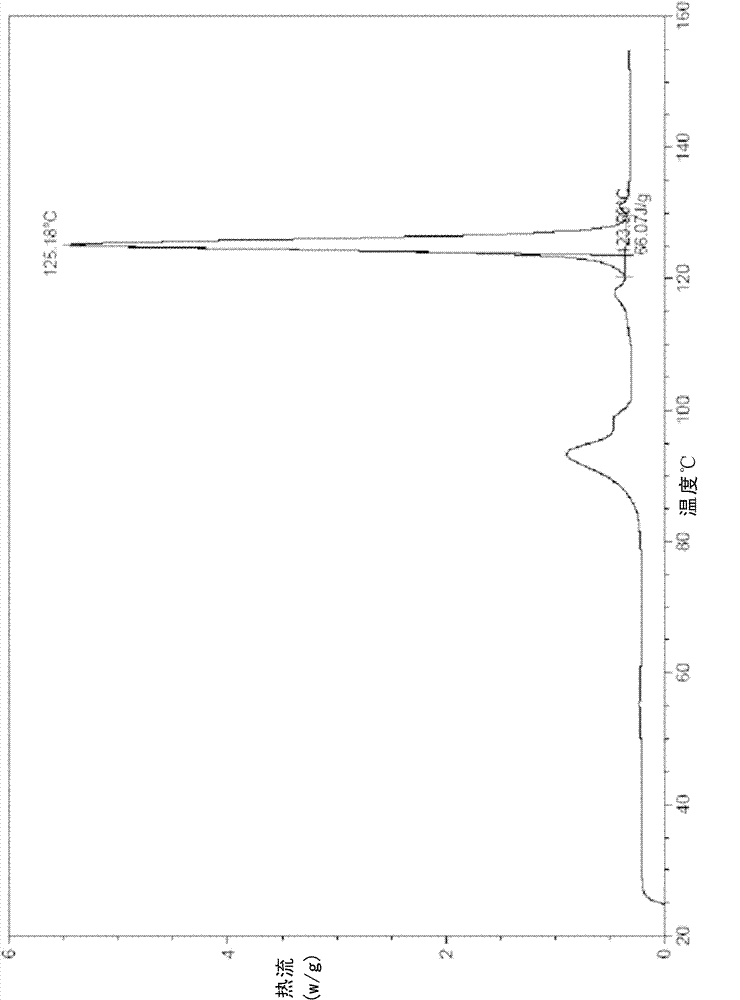
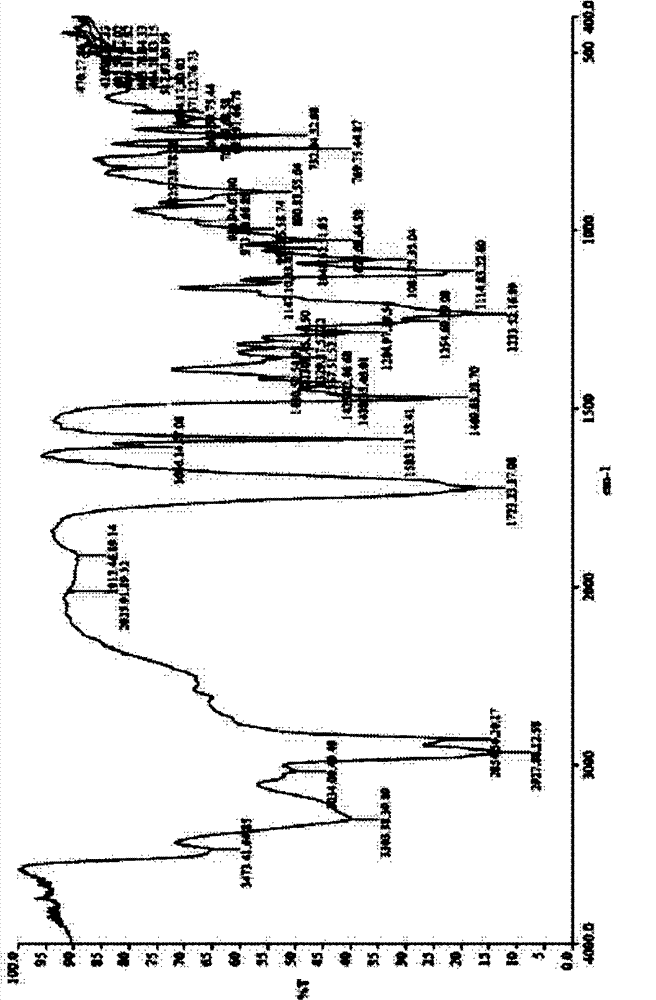
[0107] In a 25ml eggplant-shaped bottle, add the crude compound I obtained in Example 1 (1.0g) and acetone (1.5ml), heat up to 40°C and dissolve to form a homogeneous solution, then slowly cool down to 5°C and stir for 10h, filter, 5 It was washed 2-3 times with acetone at °C and dried to obtain 0.91 g of crystalline solid. X-ray powder diffraction pattern and figure 1 Consistent, Differential Scanning Calorimetry (DSC) chart with figure 2 Consistent, the infrared spectrum and image 3 Consistent, HPLC purity 99.90%. Organic residue: acetone 0.05% (mass yield: 91%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com