Crystal form of prostaglandin analogue, and preparation method and application thereof
A crystal form and drug technology, applied in the field of chemical pharmaceuticals, can solve problems such as high viscosity, excessive residual solvents, and poor stability of prostaglandin compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] The preparation method of compound I crystal form A
[0063] The present invention also provides a preparation method of crystal form A of the compound represented by formula I.
[0064] In one embodiment provided by the invention, the preparation method of the crystal form A of the compound of formula I comprises the following steps:
[0065] (1) Mix the crude product of the compound shown in formula I with solvent 1 to obtain solution 1; the solvent 1 is selected from the group consisting of acetone, butanone, methyl acetate, ethyl acetate, isopropyl acetate, or tert-butyl acetate; and
[0066] (2) Cool down and stir the solution 1 to precipitate the crystal form A of the compound of formula I.
[0067] In step (1), the mixing should be performed below the boiling point of solvent 1, preferably at 30-80°C, more preferably at 40-50°C.
[0068] In step (1), the mixing ratio (weight to volume ratio) of the crude compound represented by formula I to solvent 1 is 1:2-50...
Embodiment 1
[0112] Preparation of crude compound I
[0113] Referring to the preparation method reported in similar literature US2005209337A1, using Corey lactone and 2-oxo-3-phenoxypropyl dimethyl phosphate as starting materials, an improved Wittig condensation reaction occurs under the action of sodium hydride to obtain the key intermediate enone compound, and then through chiral reduction, tert-butyldimethylsilane protection, diisobutylaluminum hydride reduction and Wittig condensation to obtain another key intermediate, and then hydrochloric acid hydrolysis, methyl esterification and ethylenediamine aminolysis to obtain the compound I crude.
Embodiment 2
[0115] Preparation of compound I crystal form A
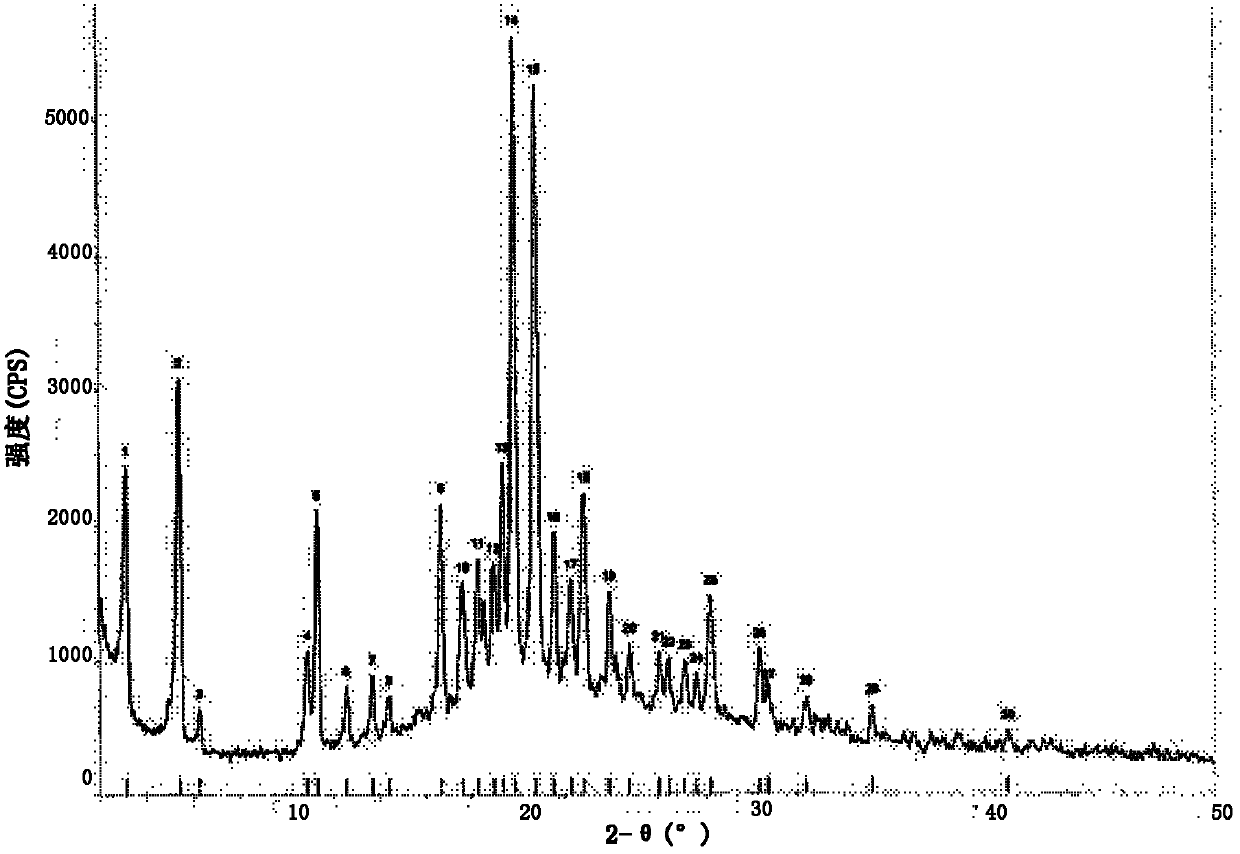
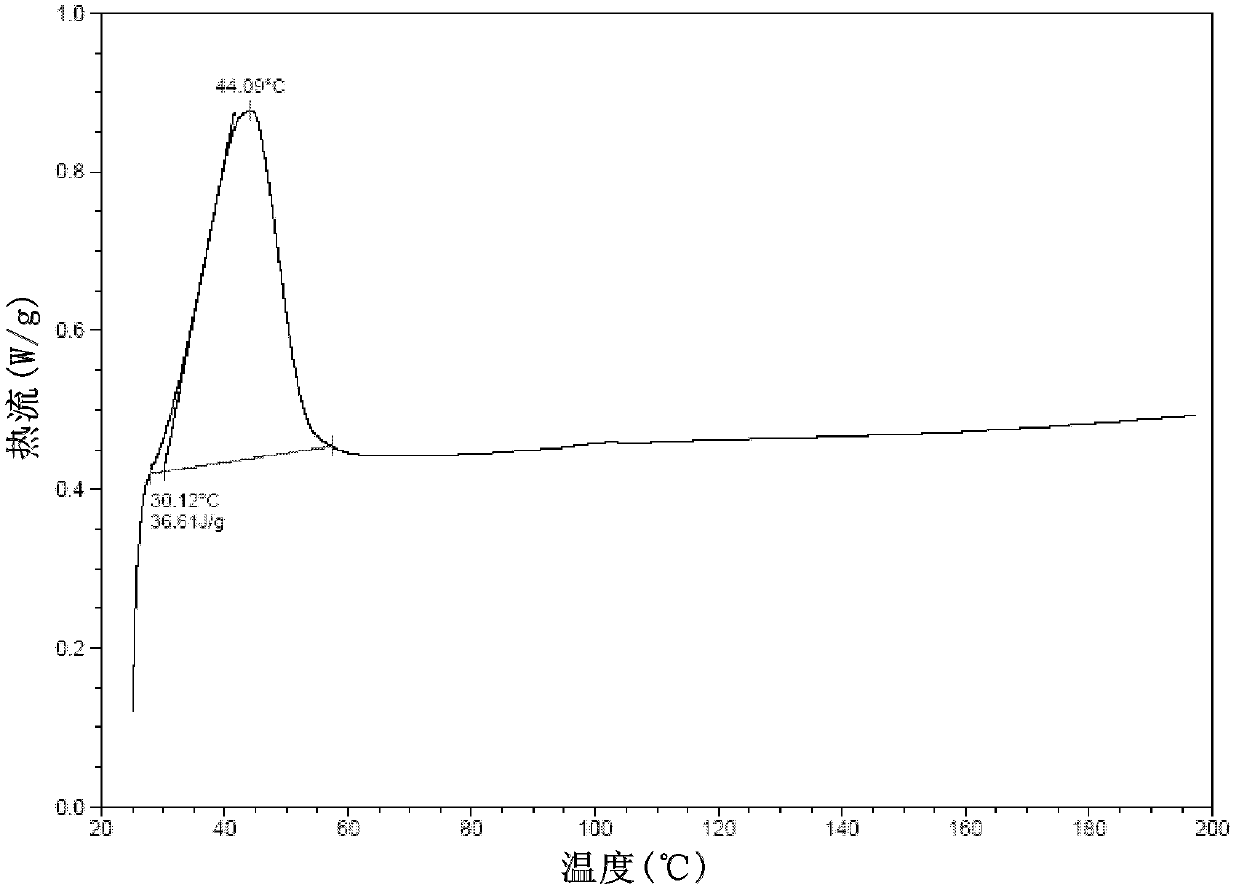
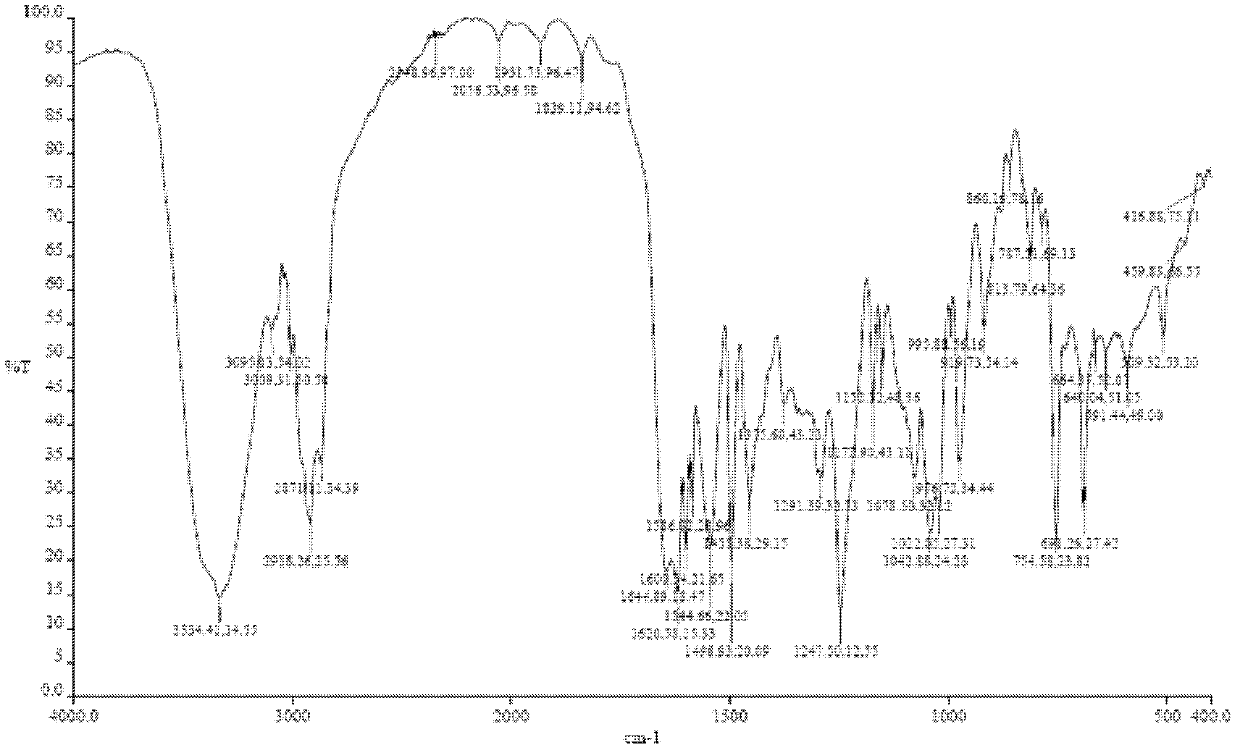
[0116] In a 50ml eggplant-shaped bottle, add the crude compound I obtained in Example 1 (2.0g) and acetone (10ml), heat up to 30°C and dissolve to form a homogeneous solution, then slowly cool down to 5°C and stir for 10h, filter, 5°C Washed 2-3 times with acetone and dried to obtain 1.22 g of crystalline solid. X-ray powder diffraction pattern see figure 1 , differential scanning calorimetry (DSC) chart see figure 2 , see the infrared spectrum image 3 , HPLC purity 99.07%, see Figure 4 . (mass yield: 61%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com