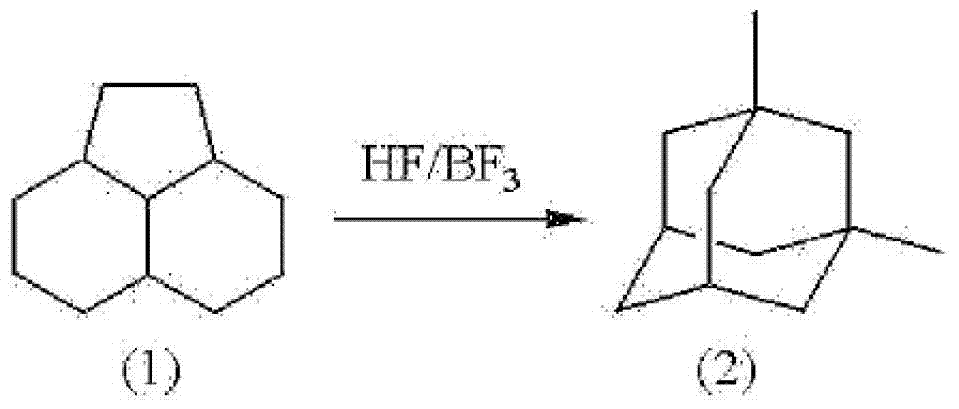
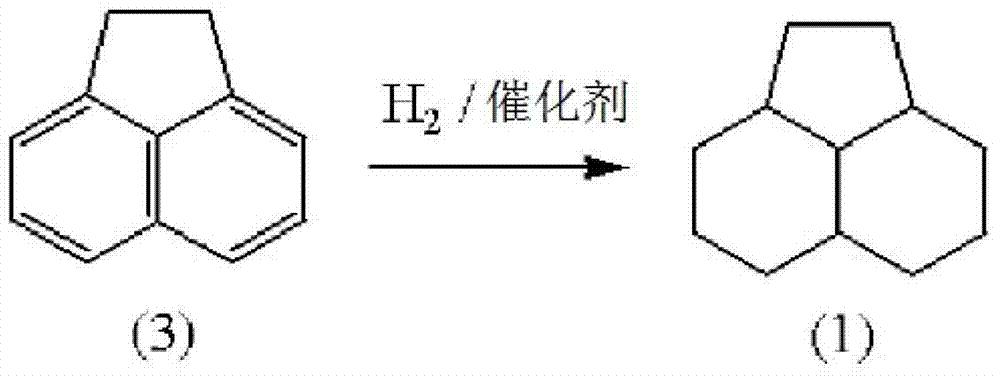
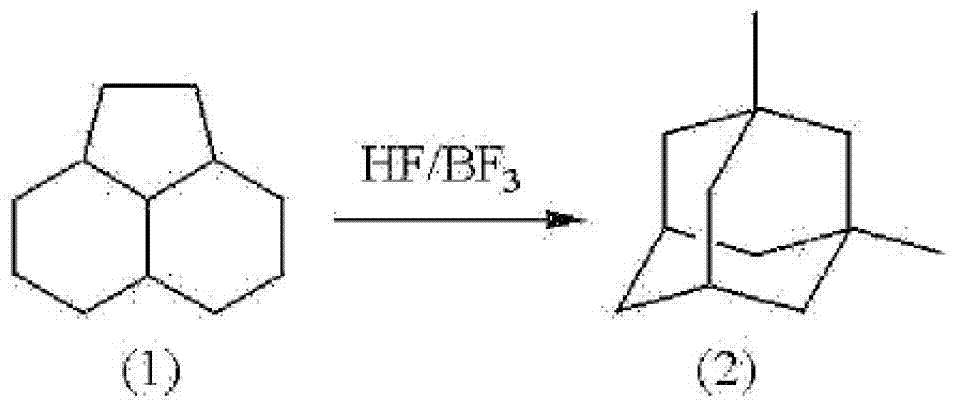Method for producing 1,3-dimethyladamantane
一种二甲基金刚烷、制造方法的技术,应用在有机化学方法、化学仪器和方法、异构化制烃等方向,能够解决不是、未知晓、不能再利用氯化铝等问题,达到高收率、降低环境负荷的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The isomerization reaction of PHA was carried out using an autoclave made of Hastelloy with an internal volume of 0.5 L having an electromagnetic stirring device, a heating device, a gas supply port, a liquid supply port, and a reactant discharge port. 50g (2.5 moles) of HF manufactured by Morita Chemical Industry Co., Ltd. and 100 g (0.6 moles) of PHA were added to the reactor, and 16 g (0.24 moles) of BF manufactured by STELLACHEMIFA CORPORATION were supplied. 3 . Next, the temperature was raised to 100° C. by a heating device without adding a solvent, and the mixture was stirred for 4 hours while maintaining the temperature. When the reaction product liquid was sampled, it was found that the yield of 1,3-dimethyladamantane was 77% based on PHA as a raw material. In addition, based on PHA as a raw material, the yield of 1-ethyladamantane as an intermediate was 15%, and no high boiling point compound was observed. Then, by standing and separating into two layers, an ...
Embodiment 2
[0053] Using HF and BF recovered in Example 1 3 , except that, the reaction was carried out under the same conditions, the yield of 1,3-dimethyladamantane was 75%, and no deactivation of the catalyst was observed. In addition, similarly to Example 1, no high boiling point compound was observed.
Embodiment 3
[0055] The isomerization reaction of PHA was carried out using an autoclave made of Hastelloy with an internal volume of 0.5 L having an electromagnetic stirring device, a heating device, a gas supply port, a liquid supply port, and a reactant discharge port. 50g (2.5 moles) of HF manufactured by Morita Chemical Industry Co., Ltd. and 100 g (0.6 moles) of PHA were added to the reactor, and 16 g (0.24 moles) of BF manufactured by STELLACHEMIFA CORPORATION were supplied. 3 . Next, the temperature was raised to 80° C. by a heating device without adding a solvent, and the mixture was stirred for 4 hours while maintaining the temperature. When the reaction product liquid was sampled, it was found that the yield of 1,3-dimethyladamantane was 51% based on PHA as a raw material. In addition, based on PHA as a raw material, the yield of 1-ethyladamantane as an intermediate was 42%, and no high boiling point compound was observed. Then, by standing and separating into two layers, an o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| rate of recovery | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| rate of recovery | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com