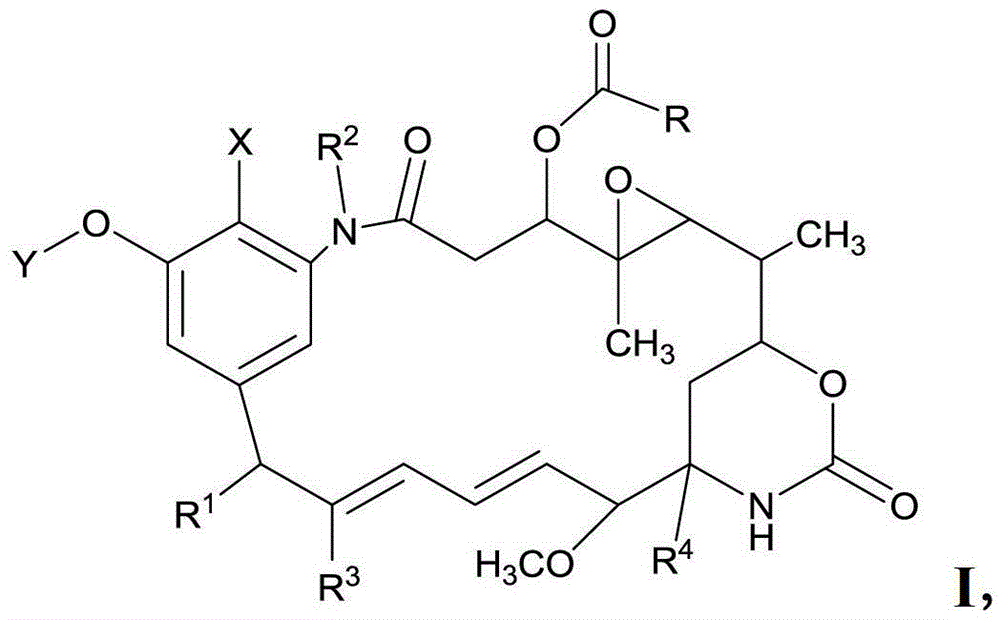
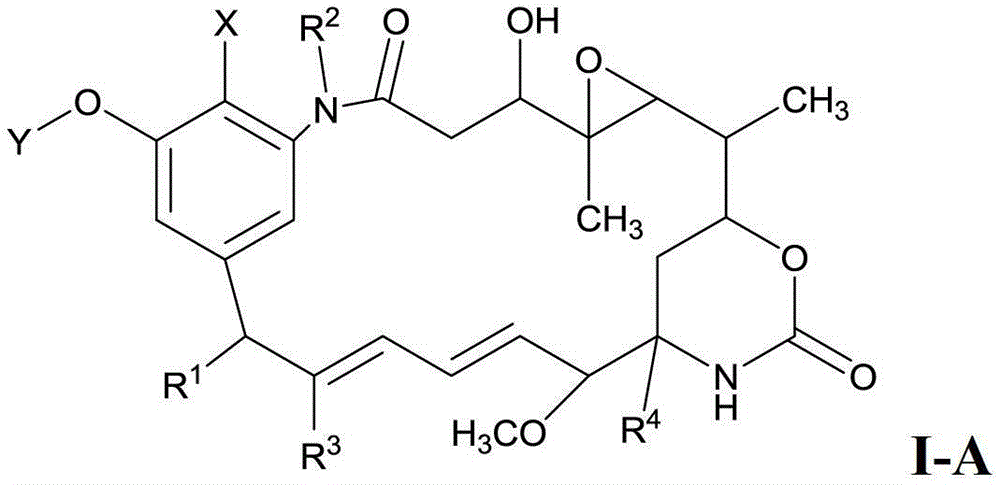
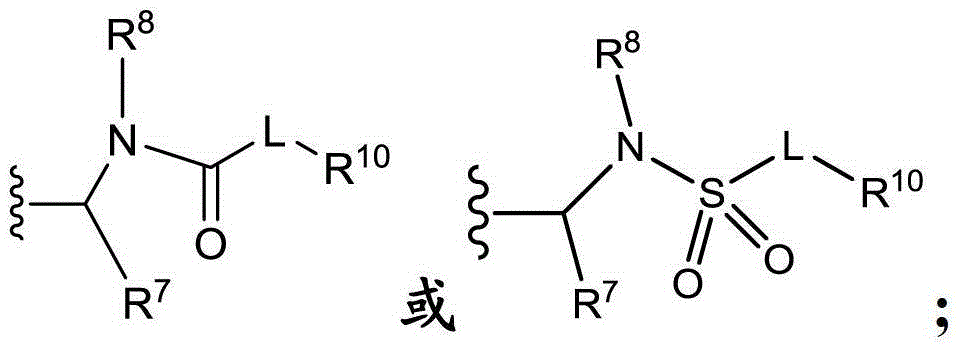
Preparation method of maytenin-like ester and composition used in method
A compound and alkyl technology, applied in the field of preparation of maytansinoid esters, can solve the problem of not giving the reaction yield and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0765] Esterification of maytansinol with acetic acid
[0766]
[0767] Weigh maytansinol (6.0mg, 0.01062mmol), acetic acid (12.75mg, 0.2124mmol), scandium triflate (3.14mg, 0.00637mmol) and DMAP (3.89mg, 0.03186mmol) into a 5ml Schlenck bottle , added dichloromethane (1 mL) under nitrogen protection, and stirred at -8°C for 0.5 hours. Add DIC (29.49mg, 0.2337mmol) dropwise, continue to stir the reaction until the reaction is complete, quench with dilute hydrochloric acid, extract with dichloromethane, wash with saturated sodium bicarbonate and saturated brine successively, dry over anhydrous sodium sulfate, spin dry solvent. Column chromatography (silica gel, CH 2 Cl 2 / MeOH30:1) to obtain the desired product ansamitocin (ansamitocin) P1. LC-MS (M+Na + ) calculated value: 629.2, measured value: 629.3.
Embodiment 2
[0769] Esterification of maytansinol with isobutyric acid
[0770]
[0771] Weigh maytansinol (6.0mg, 0.01062mmol), isobutyric acid (23.60mg, 0.2124mmol), scandium trifluoromethanesulfonate (3.14mg, 0.00637mmol) and DMAP (3.89mg, 0.03186mmol) into 5ml Schlenck bottle, add dichloromethane (1 mL) under nitrogen protection, and stir at -8oC for 0.5 hours. Add DIC (29.49mg, 0.2337mmol) dropwise, continue to stir the reaction until the reaction is complete, quench with dilute hydrochloric acid, extract with dichloromethane, wash with saturated sodium bicarbonate and saturated brine successively, dry over anhydrous sodium sulfate, spin dry solvent. Column chromatography (silica gel, CH 2 Cl 2 / MeOH30:1) to obtain the desired product ansamitocin (ansamitocin) P3. LC-MS (M+Na+) calculated: 657.3, found: 657.4.
Embodiment 3
[0773] Esterification of maytansinol with mono(2-trimethylsilylethyl) adipate
[0774]
[0775] Weigh maytansinol (6.0mg, 0.01062mmol), adipate mono(2-trimethylsilylethyl) (52.27mg, 0.2124mmol), scandium trifluoromethanesulfonate (3.14mg, 0.00637mmol) and DMAP (3.89 mg, 0.03186mmol) was placed in a 5ml Schlenck bottle, dichloromethane (1mL) was added under nitrogen protection, and stirred at -8°C for 0.5 hours. Add DIC (29.49mg, 0.2337mmol) dropwise, continue to stir the reaction until the reaction is complete, quench with dilute hydrochloric acid, extract with dichloromethane, wash with saturated sodium bicarbonate and saturated brine successively, dry over anhydrous sodium sulfate, spin dry solvent. Column chromatography (silica gel, CH 2 Cl 2 / MeOH30:1) to obtain the desired product. LC-MS (M+Na + ) calculated value: 815.3, measured value: 815.4.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap