Method for preparing taurine
A technology of taurine and sodium taurine, applied in the preparation of sulfonic acid, organic chemistry and other directions, can solve the problems of high energy consumption, high labor intensity and high production cost, and achieve energy saving, low production cost and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
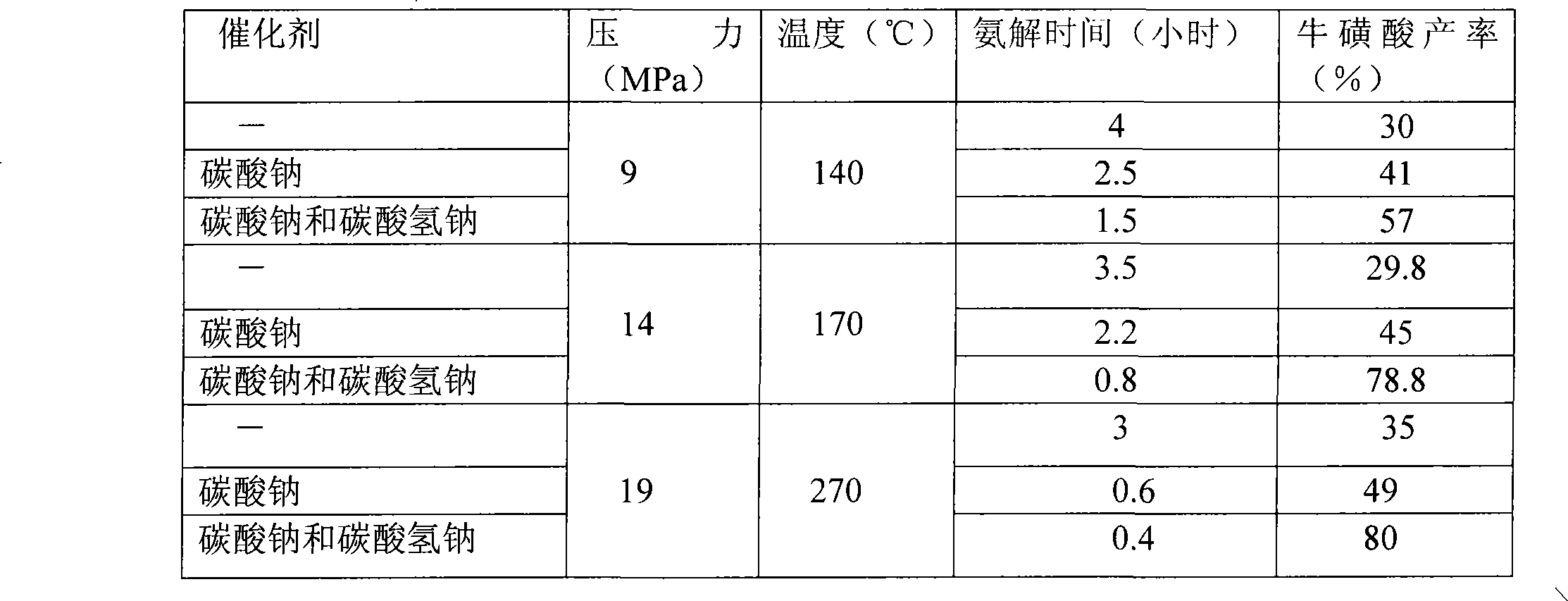
[0017] The preparation of sodium isethionate: feed ethylene oxide gas in 24wt% sodium bisulfite solution (the molar ratio of ethylene oxide and sodium bisulfite is 1:1.2) at 0.1MPa, pH Value 6.5-7.5 and react at 80°C for 1.2 hours to obtain a mixture containing sodium isethionate (99% conversion).
[0018] The preparation of sodium taurate: in the presence of catalyst sodium carbonate and sodium bicarbonate (the mol ratio of sodium carbonate and sodium bicarbonate is 3:1) (the consumption of catalyst is 1% of the quality of sodium isethionate) , Liquid ammonia is passed into the mixture containing sodium isethionate (the concentration of ammonia in the reaction solution is 20%, percent by weight) and reacted for 45 minutes at 15 MPa and 160° C. to obtain an ammonolysis solution containing sodium taurate.
[0019] Preparation of taurine: After evaporating the ammonium solution containing sodium taurine to remove ammonia, neutralize it with 98% sulfuric acid at 30-70°C to a pH v...
Embodiment 2
[0021] Preparation of sodium isethionate: react 1:1.1 molar ratio of ethylene oxide and sodium bisulfite at 0.07MPa, pH 6.5-7.5 and 75°C for 1.5 hours to prepare sodium isethionate .
[0022] The preparation of sodium taurate: in the presence of catalyst sodium carbonate and sodium bicarbonate (the mol ratio of sodium carbonate and sodium bicarbonate is 5:1) (the consumption of catalyst is 5% of the quality of sodium isethionate) , sodium isethionate and liquid ammonia (the concentration of ammonia in the reaction solution is 25%, percent by weight) reacted for 25 minutes at 21 MPa and 280° C. to obtain an ammonia solution containing sodium taurate.
[0023] Preparation of taurine: After evaporating the ammonium solution containing sodium taurine to remove ammonia, neutralize it with 95% sulfuric acid at 30-70°C to a pH value of 7.8-7.9, then concentrate, crystallize, and separate to obtain the water content In the 5-10wt% taurine wet product, the taurine wet product with a w...
Embodiment 3
[0026] Preparation of sodium isethionate: react 1:1 molar ratio of ethylene oxide and sodium bisulfite at 0.08MPa, pH 6.5—7.5 and 85°C for 1.1 hours to prepare sodium isethionate .
[0027] The preparation of sodium taurate: in the presence of catalyst sodium carbonate and sodium bicarbonate (the mol ratio of sodium carbonate and sodium bicarbonate is 4:1) (the consumption of catalyst is 3% of the quality of sodium isethionate) , Sodium isethionate and liquid ammonia (the concentration of ammonia in the reaction solution is 30%, percentage by weight) ammonolysis reaction at 17MPa and 190° C. for 30 minutes to obtain an ammonolysis solution containing sodium taurate.
[0028] Preparation of taurine: After evaporating the ammonium solution containing sodium taurine to remove ammonia, neutralize it with 95% sulfuric acid at 30-70°C to a pH value of 7.8-7.9, then concentrate, crystallize, and separate to obtain the water content In the 5-10wt% taurine wet product, the taurine wet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com