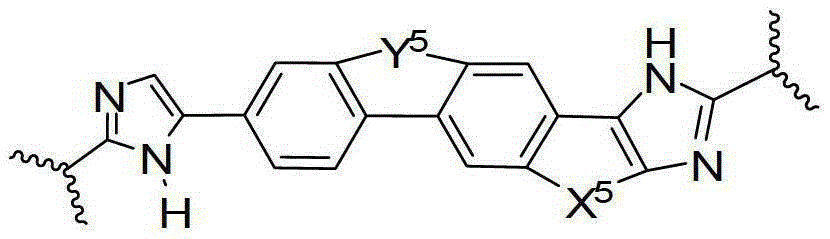
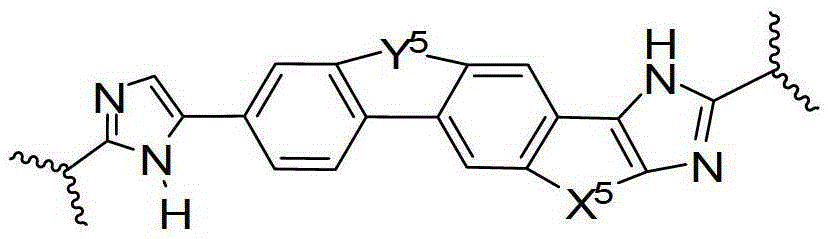
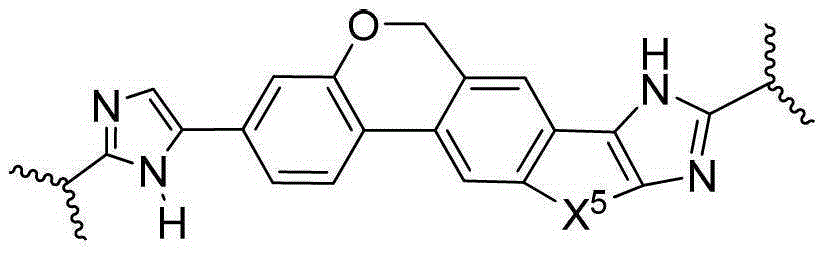
Condensed imidazolyl imidazoles as antiviral compounds
A compound and composition technology, applied in the direction of antiviral agents, compounds containing elements of group 3/13 of the periodic table, drug combinations, etc., can solve problems such as restricted use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0241] In one embodiment, the present disclosure provides a compound, or a pharmaceutically acceptable salt or prodrug thereof, said compound having the general formula:
[0242]
[0243] Wherein the imidazole rings shown in the general formulas A1, A2, A3 and A4 are optionally substituted by one or more groups independently selected from halogen, haloalkyl, cyano or alkyl.
[0244] In one embodiment, the present disclosure provides a compound, or a pharmaceutically acceptable salt or prodrug thereof, said compound having the general formula:
[0245]
[0246] Wherein the imidazole rings shown in the general formulas A2 and A4 are optionally substituted by one or more groups independently selected from halogen, haloalkyl, cyano or alkyl.
[0247] In one embodiment, E 1a and E 1b At least one of is -N(H)(alkoxycarbonyl).
[0248] In one embodiment, E 1a and E 1b At least one of is -N(H)C(=O)OMe.
[0249] In one embodiment, E 1a and E 1bBoth are -N(H)C(=O)OMe.
[...
Embodiment LQ
[0302]
[0303]
[0304] 7-(2-Bromo-5-chlorobenzyloxy)-3,4-dihydronaphthalen-1(2H)-one
[0305] 7-Hydroxy-1-tetralone (13.9 g, 85.7 mmol) and 1-bromo-2-(bromomethyl)-4-chlorobenzene (25.6 g, 90.0 mmol) was added potassium carbonate (24 g, 172 mmol). The reaction was stirred under argon for 18 hours, then diluted with ethyl acetate (1 L). The organics were washed three times with water and once with brine. The organic layer was then dried over magnesium sulfate, filtered and concentrated. To the resulting oil was added methanol (500 mL), and the suspension was stirred for thirty minutes. 7-(2-Bromo-5-chlorobenzyloxy)-3,4-dihydronaphthalen-1(2H)-one (27.8 g, 89% yield) was isolated by filtration.
[0306] 3-Chloro-10,11-dihydro-5H-dibenzo[c,g]chromen-8(9H)-one
[0307] To a solution containing palladium(II) pivalate (1.18g, 3.8mmol), tris(4-fluorophenyl)phosphine (1.20g, 3.8mmol), pivalic acid (2.33g, 22.8mmol) and potassium carbonate ( 31.8 g, 228 mmol) in a 1 L fla...
Embodiment L
[0322]
[0323] (2S,4S)-1-tert-butyl 2,4-dimethylpyrrolidine-1,2,4-tricarboxylate
[0324] To a solution of (2S,4S)-1-tert-butyl 2-methyl 4-cyanopyrrolidine-1,2-dicarboxylate (9.0 g, 35.4 mmol) in MeOH (196 mL) was added HCl ( 4M in 1,4-dioxane, 100 mL, 403 mmol). The solution was stirred at room temperature for 16 h, and concentrated in vacuo. The crude intermediate was dissolved in EtOAc (180 mL) and basified with aqueous bicarbonate (sat.). Di-tert-butyl bicarbonate (8.5 g, 38.9 mmol) was added and the biphasic solution was stirred at room temperature for 12 h. The layers were then separated, and the aqueous layer was back extracted with EtOAc. The combined organic layers were washed with brine, passed through Na 2 SO 4 Dried and concentrated. The crude oil was purified by silica gel chromatography (15% to 40% to 100% EtOAc / hexanes) to provide (2S,4S)-1-tert-butyl 2,4-dimethylpyrrolidine-1,2,4 - Tricarboxylates (9.56 g, 94%).
[0325] (3S,5S)-1-(tert-butoxycarbon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com