Novel capsule filled with alogliptin solid preparation and pioglitazone solid preparation
A technology of alogliptin and pioglitazone, which is applied in the field of oral hard capsules, can solve the problems of reducing effects, affecting qualitative and quantitative analysis results, increasing side effects, etc., and achieves the effects of improved stability, easy inspection and quality control, and enhanced compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
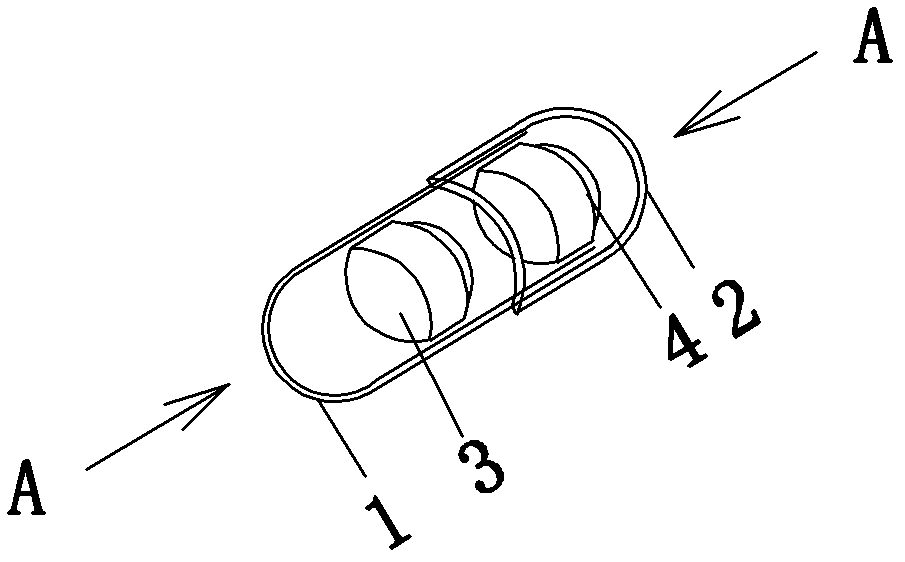
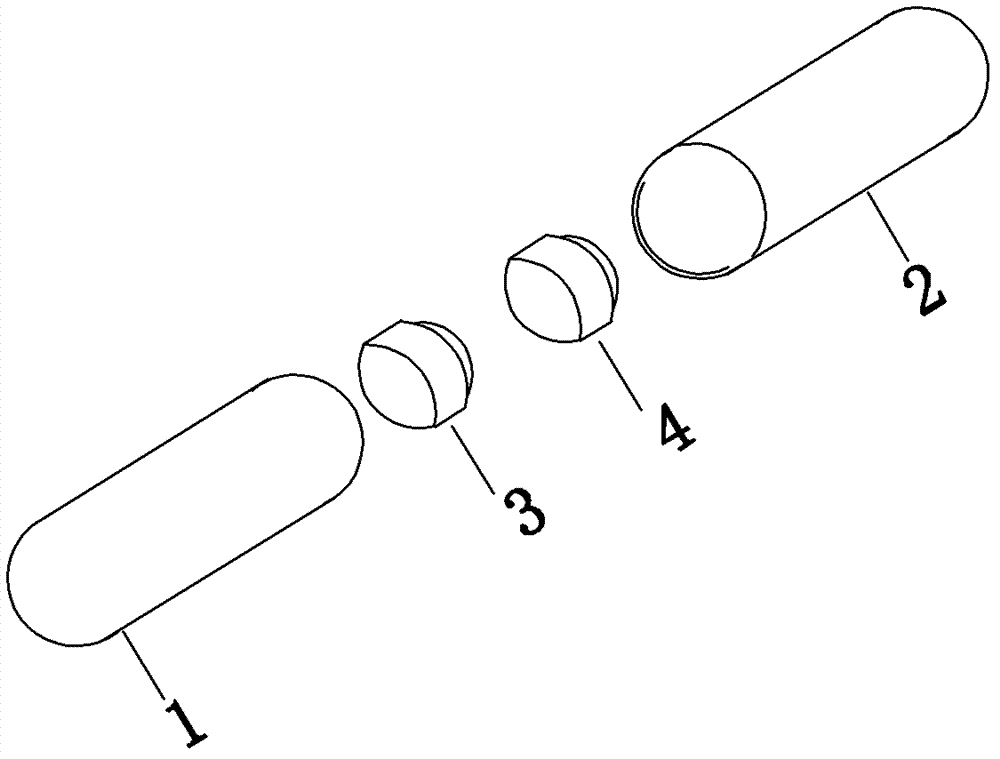
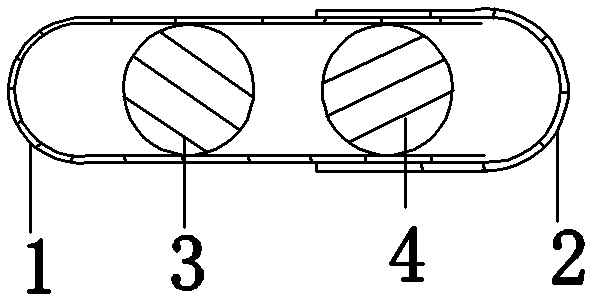
[0018] Embodiment 1: see attached Figure 1-3 As shown, a tablet capsule filled with one pioglitazone tablet; one alogliptin tablet. The capsule specification is size 0. The capsule shell is composed of a lower capsule body 1 and an upper capsule body 2 set, which are equipped with the above two cylindrical tablets coated with different colors, namely alogliptin tablets (25mg alogliptin), pioglitazone tablets (containing pioglitazone 15mg ).
[0019] Pioglitazone tablets are yellow gastric-soluble film-coated, and alogliptin tablets are red film-coated. The capsules come in a transparent pp bottle packaging.
Embodiment 2
[0020] Example 2: The only difference from Example 1 is the dose, in which alogliptin tablets (alogliptin 12.5 mg) and pioglitazone tablets (containing pioglitazone 7.5 mg) are included.
[0021] The following will be explained in conjunction with the test example:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com