Method for preparing beta-lactam derivative through enzymatic reaction
A technology of enzymatic reaction and synthesis method, applied in the direction of fermentation, etc., can solve the problems of harsh reaction conditions, complicated operation, high toxicity of reagents, etc., and achieve the effect of mild reaction conditions, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
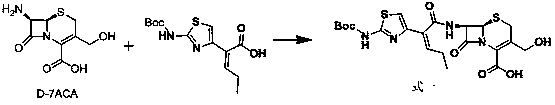
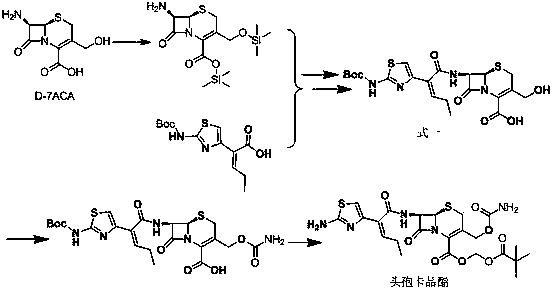
[0025] Reference Example 1: 7β-[(Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-butenoyl]amino-3-acetoxymethyl-3-cephem-4- Synthesis of carboxylic acid (Formula 2) (refer to US4500716)
[0026] Add 142g (0.5mol) of (Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-butenoic acid and 76mL (0.55mol) of triethylamine into dichloromethane (400mL) and cool down To -50°C, 40mL (0.52mol) of methanesulfonyl chloride was added dropwise, the temperature was controlled between -50°C to -40°C, and the drop was completed, and stirred at -50°C to -40°C for 5h. Then, add 163g (0.6mol) of 7-ACA (0.6mol) and 180mL (1.3mol) of triethylamine in dichloromethane (400mL) dropwise to the reaction solution, and control the temperature between -50°C and -40°C. ℃~-40℃ for 3h. Acidify with dilute hydrochloric acid and extract with ethyl acetate. The extract was washed with brine and dilute sodium bicarbonate water, dried, concentrated, and solidified to obtain 7β-[(Z)-2-(2-tert-butoxycarbonylaminothia...
Embodiment 1
[0027] Example 1: 7β-[(Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-butenoyl]amino-3-hydroxymethyl-3-cephem-4-carboxy Synthesis of acid (Formula 1)
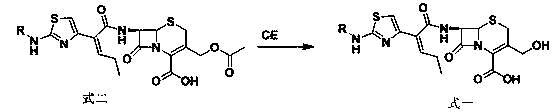
[0028] Add 40g (72.4mmol) of compound formula 2 into 240mL of purified water to make the concentration of formula 2 300mM, adjust the pH to 7.8 with 25% ammonia water to completely dissolve the compound of formula 2, add immobilized deacetylesterase 2KU, and react at 25°C for 6h . After suction filtration, the pH of the filtrate was adjusted to 2-5 with dilute hydrochloric acid, and a white solid was precipitated. After suction filtration and drying, 34.6 g of Compound 1 was obtained, with a yield of 94%.
Embodiment 2
[0029] Example 2: 7β-[(Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-butenoyl]amino-3-hydroxymethyl-3-cephem-4-carboxy Synthesis of acid (Formula 1)
[0030] Add 40g (72.4mmol) of compound formula 2 into 60mL of purified water and 60mL of acetone to make the concentration of formula 2 600mM, adjust the pH to 7 with 25% ammonia water to completely dissolve the compound of formula 2, add immobilized deacetylesterase 1KU, 15 ℃ reaction 10h. After suction filtration, the pH of the filtrate was adjusted to 3-4 with dilute hydrochloric acid, and a white solid was precipitated, which was filtered by suction and dried to obtain 33.2 g of Compound 1 with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com