A kind of synthetic method and application of flavonoids
The technology of a flavonoid compound and a synthesis method, which is applied in the field of medicinal chemistry synthesis, can solve the problems of low yield, lack of 5-hydroxy flavonoids, troublesome separation and purification, etc., and achieves the effects of cheap and easy-to-obtain raw materials and wide adaptability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
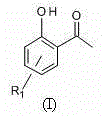
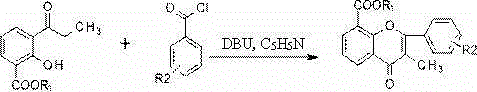
[0052] Take 11.0 g (0.1 mol) of resorcinol, 15.4 mL of glacial acetic acid, and 16.3 g (0.12 mol) of dry zinc chloride, mix them, heat in an oil bath to 140.0-150.0°C, and stir for 2.0 h under reflux. TLC monitored the completion of the reaction, cooled to room temperature, poured the reaction solution into ice water to cool, and suction filtered. The filter cake was washed several times with dilute hydrochloric acid to remove zinc salts, and an orange-yellow solid was obtained. The solid was heated and dissolved with dilute hydrochloric acid, decolorized with activated carbon, and then recrystallized repeatedly with dilute hydrochloric acid to finally obtain orange-yellow crystals of 2,4-dihydroxyacetophenone (compound 2, 10.0 g, 0.0657 mol).
[0053] Add 2,4-dihydroxyacetophenone (2.46 g, 16.2 mmol) to the reaction flask, anhydrous K 2 CO 3(22.69g, 162.0 mmol), and dry acetone (60 mL), refluxed in an oil bath at 60°C for 10 min, and added anhydrous pyridine (1.28g, 16.2 mm...
Embodiment 2~13
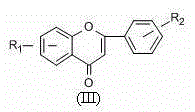
[0061] Synthesis of other flavonoids
[0062] The synthesis steps of other flavonoids were carried out according to the synthesis steps of 7-hydroxyflavone in Example 1.
[0063] Wherein, used reactant and target product are as follows:
[0064] .
[0065] The consumption and reflux condition of each raw material in each embodiment are as follows:
[0066] .
[0067] The character of each product of embodiment 2~13 gained
[0068] 4′-Fluoro-7-hydroxyflavone: yellow needle crystal, the yield is about 95.7%, melting point: 250~254 ℃; 1 H NMR (400 MHz, Acetone) δ 9.74 (s, 1H,-OH), 8.29 – 8.12 (m, 2H,2′, 6′-H), 8.03 (d, J = 8.7 Hz, 1H, 5-H), 7.42 (t, J = 8.8 Hz, 2H,3′, 4′- H), 7.12 (d, J = 2.2 Hz, 1H,8-H), 7.05 (dd, J = 8.7, 2.3 Hz, 1H,6-H), 6.79 (s, 1H,3-H); ESI-MS (positive): calcd for C 15 h 9 o 3 F([M+Na] + ) 257.24; found 257.00(M+H) + .
[0069] 4′-Chloro-7-hydroxyflavone: white needle crystal, the yield is about 92.7%, melting point: 284.9~286℃; 1 H ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com