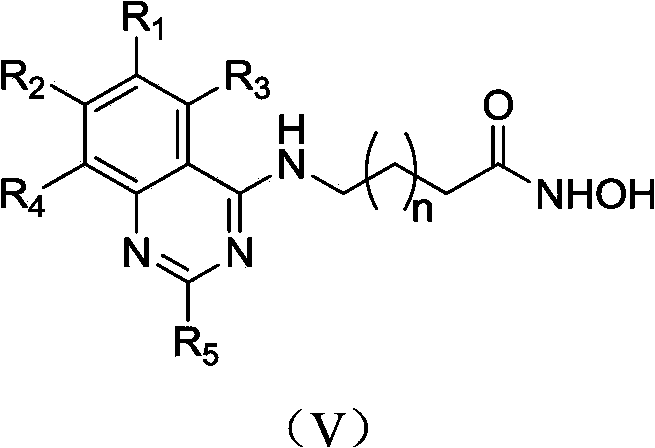
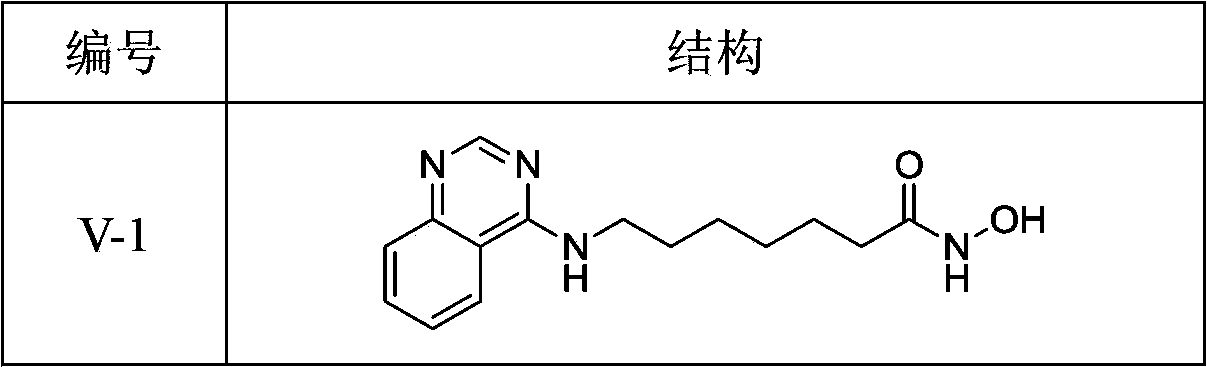
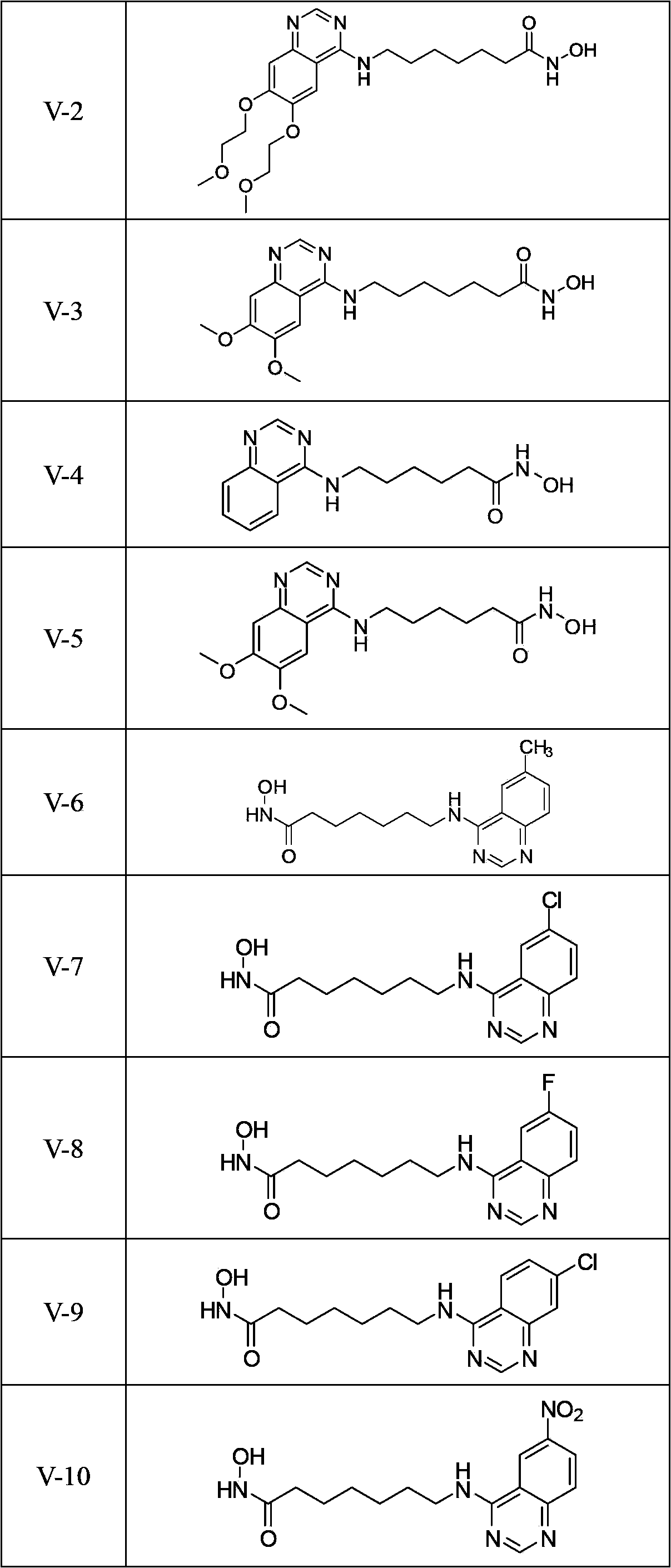
4-aminoquinazolinehydroxamic acid compounds and applications thereof as antitumor medicaments
A technology of aminoquinazoline hydroxime and compound, which is applied in the application field of antineoplastic drugs, can solve problems such as limited therapeutic range, cell death, and adverse reactions, and achieve good selective inhibitory activity, weak inhibitory effect, and good inhibitory activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Ⅴ-1 Synthesis of N-hydroxy-7-(quinazoline-4-amino)heptanamide
[0077] 4-Chloroquinazoline (0.164g, 1mmol), 7-aminoheptanoic acid methyl ester hydrochloride (0.21g, 1mmol), KOH (0.14g, 2.5mmol), hydroxylamine hydrochloride (0.7g, 2.5mmol) as raw materials, Ⅴ-1 was synthesized according to the general synthesis method of class V compounds, and the yield was 55.9%.
[0078] ESI-MS[M+H] + :m / z 289.1695
[0079] 1 H NMR (400MHz, DMSO-d 6 )δppm: 1.30(m, 4H), 1.48(m, 2H), 1.60(m, 2H), 1.70-1.80(m, 3H), 1.95(t, 1H, J=8.0Hz), 3.52(m, 2H ),7.49(t,1H,J=8.0Hz),7.66(d,1H,J=8.0Hz),7.75(t,1H,J=8.0Hz),8.27(d,1H,J=8.0Hz), 8.34(s,1H),8.43(s,1H)
Embodiment 2
[0081] Ⅴ-2 Synthesis of N-hydroxy-7-(6,7-dimethoxyethoxyquinazoline-4-amino)heptanamide
[0082] 6,7-dimethoxyethoxy-4-chloroquinazoline (0.312g, 1mmol), methyl 7-aminoheptanoate hydrochloride (0.21g, 1mmol), KOH (0.14g, 2.5mmol), Hydroxylamine hydrochloride (0.7g, 2.5mmol) was used as a raw material, and V-2 was synthesized according to the general synthesis method of class V compounds, with a yield of 65.3%.
[0083] ESI-MS[M+H] + :m / z 437.2449
[0084] 1 H NMR (400MHz, DMSO-d 6 )δppm: 1.32(m, 4H), 1.42-1.63(m, 4H), 1.96(t, 2H, J=8.0Hz), 2.21(t, 1H, J=8.0Hz), 3.34-3.51(m, 7H ),3.73(s,6H),4.25(m,4H),7.15(s,1H),7.91(s,1H),8.38(s,1H),8.64(s,1H)
Embodiment 3
[0086] Ⅴ-3 Synthesis of N-hydroxy-7-(6,7-dimethoxyquinazoline-4-amino)heptanamide
[0087]6,7-dimethoxy-4-chloroquinazoline (0.224g, 1mmol), methyl 7-aminoheptanoate hydrochloride (0.21g, 1mmol), KOH (0.14g, 2.5mmol), hydroxylamine hydrochloride ( 0.7g, 2.5mmol) as raw materials, and synthesized V-3 according to the general synthesis method of class V compounds, with a yield of 66.0%.
[0088] ESI-MS[M+H] + :m / z 349.32
[0089] 1 H NMR (400MHz, DMSO-d 6 )δppm: 1.32(m, 4H), 1.49(m, 2H), 1.61(m, 2H), 1.69(s, 1H), 1.96(t, 2H, J=8.0Hz), 3.48-3.57(m, 3H ),3.88(s,6H),7.06(s,1H),7.66(s,1H),8.06(s,1H),8.32(s,1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com