A pharmaceutical composition for treating vomiting
A composition and drug technology, applied in the field of medicine, can solve the problems of delayed or early vomiting that are not particularly effective and cannot prevent nausea and vomiting of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
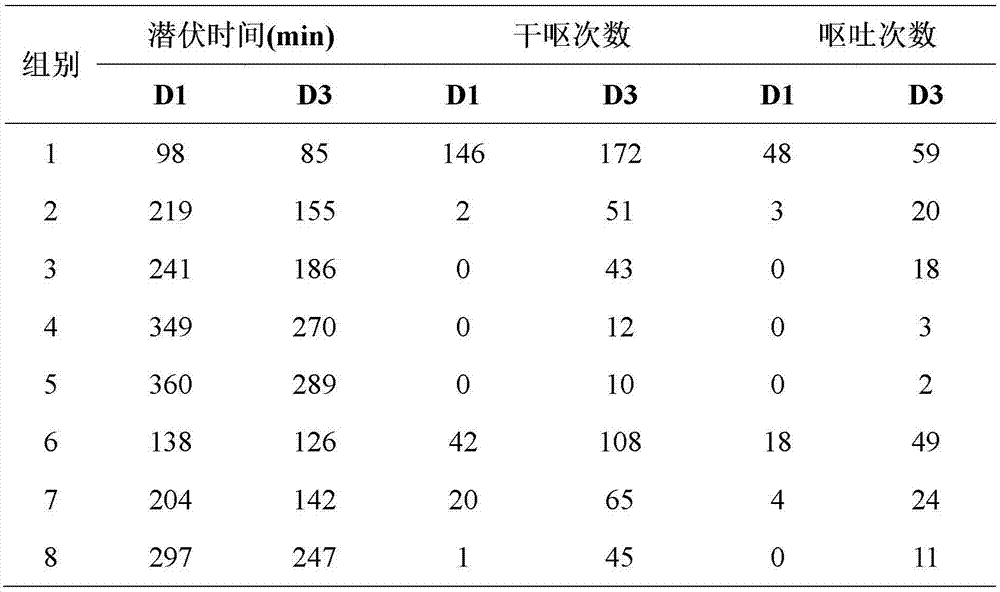
Examples
Embodiment 1
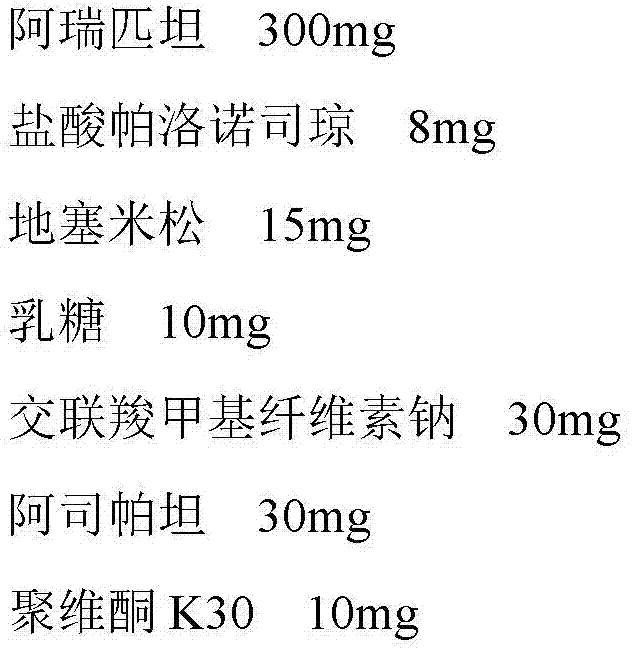
[0012] Example 1: Aprepitant / Palonosetron Hydrochloride / Dexamethasone Granules
[0013] The prescription is as follows, all of which are the weight composition of the unit preparation:
[0014]
[0015] The preparation method is as follows:
[0016] Pass aprepitant, palonosetron hydrochloride, dexamethasone, lactose, croscarmellose sodium, aspartame, and povidone K30 through an 80-mesh sieve, and then weigh them according to the prescription amount The amount of 1000 unit preparations, mix thoroughly and evenly. Add 40ml of water, adjust the stirring rate of the wet mixing granulator to 160 rpm, the shear rate to 2000 rpm, granulate for 10 minutes, pass through a 16-mesh sieve, dry at 70°C and granulate with a 14-mesh sieve to obtain .
Embodiment 2
[0017] Embodiment 2: Aprepitant / palonosetron hydrochloride / dexamethasone dispersible tablet
[0018] The prescription is as follows, all of which are the weight composition of the unit preparation:
[0019]
[0020]
[0021] The preparation method is as follows:
[0022] Weigh aprepitant, palonosetron hydrochloride, and dexamethasone according to the prescription amount, use microcrystalline cellulose as filler, cross-linked carmellose sodium, polyvinylpyrrolidone as disintegrant, 5% PVP The 60% alcohol solution is used as the binder, and the micropowdered silica gel is used as the flow aid. It is granulated in one step in a fluidized bed, and then compressed into tablets.
Embodiment 3
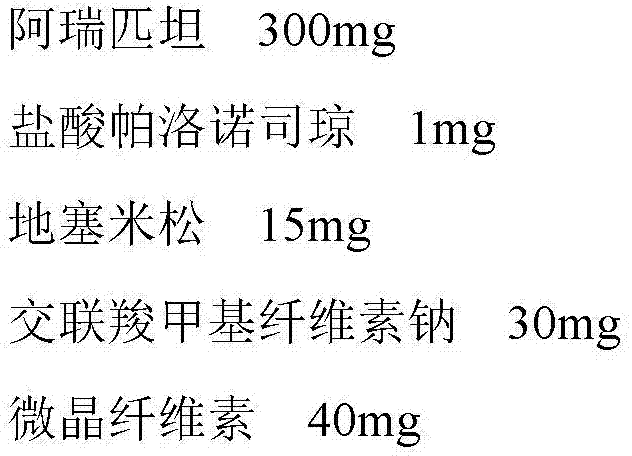
[0023] Embodiment 3: Aprepitant / palonosetron hydrochloride / dexamethasone capsule
[0024] The prescription is as follows, all of which are the weight composition of the unit preparation:
[0025]
[0026] The preparation method is as follows:
[0027] Aprepitant, panolosetron hydrochloride, dexamethasone, microcrystalline cellulose, and magnesium stearate were respectively passed through a 100-mesh sieve. After weighing according to the prescription amount, put it in a stirring mixer and stir for 15 minutes, fill it into an empty capsule shell, and obtain.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com